Radiation-induced angiosarcoma of the breast
Images

CASE SUMMARY
Our patient is a 45-year-old female with a past medical history of T2N1M0 (stage IIb, grade 3), ER positive, PR negative, HER2/NEU positive infiltrating ductal carcinoma of the left breast at the age of 38 treated with left modified radical mastectomy transverse rectus abdominis muscle (TRAM) flap reconstruction, and later with a latissimus flap following the development of a thrombus in the original TRAM flap. Following surgical therapy, she received doxorubicin and cyclophosphamide, followed by paclitaxel and trastuzumab. In addition, she received chest wall radiation.
Approximately six years later, during a routine surveillance visit, she was found to have small nodules on her chest wall inferior to her latissimus flap. These nodules progressed rapidly, eventually encompassing over 50% of the inferior aspect of the native breast, chest wall fold, latissimus dorsi flap, and skin over the xiphoid. The rest of the physical exam and laboratory findings were unremarkable.
IMAGING FINDINGS
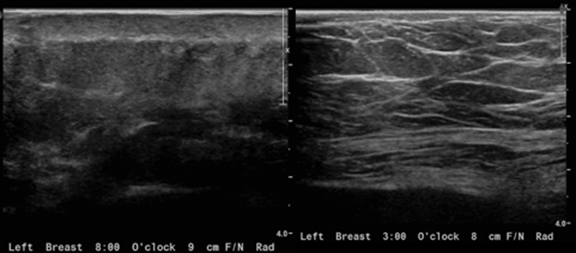
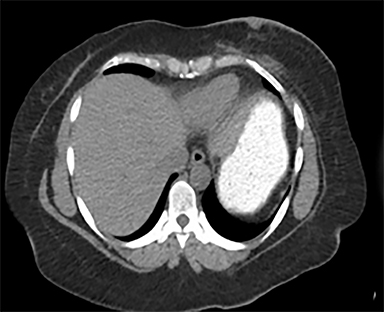
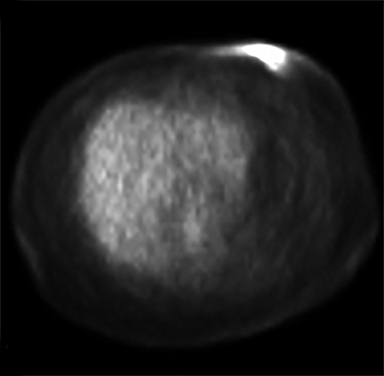
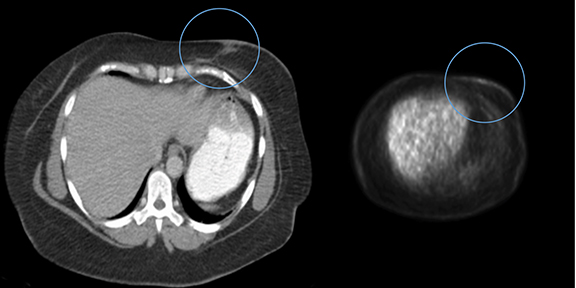
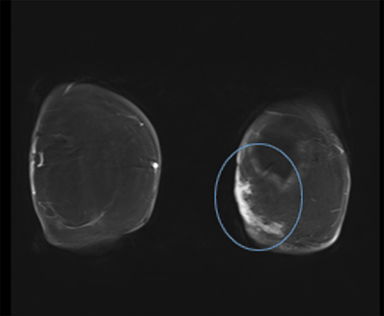
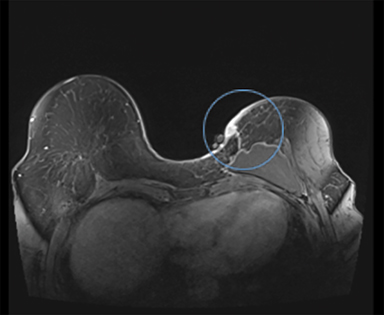
Diagnostic workup included a mammogram, breast sonogram, breast MRI and PET-CT scan. The mammogram did not reveal any suspicious findings. Sonography of the left breast showed thickening of the skin in the region of the cutaneous nodularity (Figure 1). No other site of abnormal increased uptake was visualized in the body (Figures 2 and 3). PET scan revealed a large area of curvilinear configuration of diffuse, intense uptake in the superficial and medial inferior aspect of the reconstructed left breast, fusing to thickened skin and subcutaneous tissue, with a maximum standardized uptake value of 15. MRI of the thorax demonstrated diffuse nodular thickening and enhancement of the skin, primarily along the medial inferior aspect of the left reconstructed breast and chest wall. The nodular enhancement extended into the soft tissues deep to the skin at the level of the inferior edge of the implant, measuring 4 × 1.2 × 2 centimeters. Superior to this enhancement, another area of nodular enhancement was seen abutting the skin, measuring 1.6 × 3 × 1.6 centimeters. No involvement of the muscle was visualized. Small areas of nodular skin enhancement were also noted on the lateral aspect of the breast (Figure 4).
DIAGNOSIS
Punch biopsy of two sites in the region of nodular skin thickening revealed a grade 2 angiosarcoma.
DISCUSSION
Angiosarcoma is a type of rare, rapidly proliferating, soft tissue sarcoma (STS) derived from anaplastic endothelial cells lining the blood vessel walls. They make up 4.1% of STS, which comprise 1% of all malignant tumors.1 Angiosarcomas are becoming recognized as a complication following radiation therapy in women receiving conservative therapy for breast cancer treatment, as well as lymphedema following axillary node dissection.1,2 As the incidence of breast cancer in the western world rises, so is the incidence of radiation induced angiosarcoma of the breast (RIASB), with a cumulative 15-year incidence of 0.9/1,000 breast cancer patients receiving radiation as a means of conservative therapy, with an average age of onset of 68 years.2,3 Overall survival for post-irradiation breast sarcomas are poor, with a mean 5-year survival of 27-35%.3
RIASB typically presents with nonspecific clinical findings, such as skin thickening and discoloration, fibrosis, telangiectasia, and nodularity.4 The guidelines for making the diagnosis of radiation induced sarcoma (RIS) are often applied to RIASB.5,6 Pathologic findings include a poorly defined hemorrhagic mass, and histologic findings may include anastomosing vascular channels within the breast parenchyma that are lined by malignant endothelial cells containing small and pale nuclei.7 Treatment generally consists of surgical resection with mastectomy, and chemotherapy to reduce the risk of recurrence.7
Imaging generally has poor specificity for detecting RIASB. Mammography is used routinely for breast cancer surveillance. However, it is nonspecific for RIASB and may show an ill-defined, noncalcified mass or focal asymmetry, or appear normal.7-8 It does not appear that mammography is beneficial in the initial detection of, or monitoring for the recurrence of RIASB.
If sonographic findings are present, these may appear as a hypoechoic, hyperechoic, or heterogeneous mass, with or without acoustic shadowing.7 Sonograms may be useful as an initial screening modality at sites of previous radiation or tumor location, as well as for comparison, should patients develop clinically significant abnormalities such as skin nodularity at a site of previous radiation.
Magnetic resonance imaging (MRI) has shown promise in the diagnosis of RIASB. Kikawa et al demonstrated findings that may be specific for angiosarcomas, including marked hyperintensity on T2-weighted images,prolongation of enhancement on the dynamic phase, and the presence of multiple regions without enhancement in the tumor.9 MRI may be a useful imaging technique to detect lower grade angiosarcomas before metastases and tumor growth, as well as delineate ambiguities in breast masses.
FDG-PET/CT is useful for determining whether or not the lesion in question is malignant, as well as localizing its location. Combining the two imaging modalities has been shown to improve both sensitivity and specificity for detecting loco-regional occurrence and recurrence, anatomical correction for the poor spatial resolution on the FDG-PET modality, and differentiation between benign and malignant lesions.10 This data lends support that FDG-PET/CT is a useful imaging modality for further evaluation of patients that develop clinically significant changes at the site of previous radiation therapy.
CONCLUSION
Although rare, due to the poor prognosis, RIASB remains a concern as radiation therapy is an acceptable and highly utilized therapy for breast cancer. The tumors may grow and metastasize rapidly by the time physical signs are noticed by the patient or physician, and they have high recurrence rates. While data and standardized guidelines are still lacking for RIASB surveillance, a greater frequency of clinician visits, high index of suspicion, early and routine imaging studies utilizing MRI and PET/CT, and breast biopsies may be beneficial in earlier detection and treatment of this tumor.
REFERENCES
- Toro J, Travis L, Wu H, Zhu K, Fletcher C, Devesa S. Incidence patterns of soft tissue sarcomas, regardless of primary site, in the surveillance, epidemiology and end results program, 1978–2001: An analysis of 26,758 cases. International Journal of Cancer. 2006;119(12):2922-2930.
- Stokkel M, Peterse H. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69(12):2965-2968.
- Yap J, Chuba P, Thomas R, Aref A, Lucas D, Severson R et al. Sarcoma as a second malignancy after treatment for breast cancer. International Journal of Radiation Oncology*Biology*Physics. 2002;52(5):1231-1237.
- Wijnmaalen A, Van Ooijen B, Van geel B, Henzen-Logmans S, Treurniet-Donker A. Angiosarcoma of the breast following lumpectomy, axillary lymph node dissection, and radiotherapy for primary breast cancer: Three case reports and a review of the literature. International Journal of Radiation Oncology*Biology*Physics. 1993;26(1):135-139.
- Cahan W, Woodard H, Higinbotham N, Stewart F, Coley B. Sarcoma in irradiated bone. Report of eleven cases. Cancer. 1948;1(1):3-29.
- Arlen M, Higinbotham N, Huvos A, Marcove R, Miller T, Shah I. Radiation-induced sarcoma of bone. Cancer. 1971;28(5):1087-1099.
- Glazebrook K, Magut M, Reynolds C. Angiosarcoma of the Breast. American Journal of Roentgenology. 2008 190:2, 533-538 PMID: 18212243
- Liberman L, Dershaw D, Kaufman R, Rosen P. Angiosarcoma of the breast. Radiology. 1992;183(3):649-654.
- Kikawa Y, Konishi Y, Nakamoto Y, Harada T, Takeo M, Ogata M et al. Angiosarcoma of the Breast – specific findings of MRI. Breast Cancer. 2006;13(4):369-373.
- Al-Ibraheem A, Buck A, Benz M, Rudert M, Beer A, Mansour A et al. 18 F-Fluorodeoxyglucose positron emission tomography/computed tomography for the detection of recurrent bone and soft tissue sarcoma. Cancer. 2012;119(6):1227-1234.
Citation
E B, P G, D L. Radiation-induced angiosarcoma of the breast. Appl Radiol. 2018;(3):31A-31C.
March 8, 2018