Quality and Safety in Medical Imaging During Pregnancy and Lactation — Part 2
CME credits are available here.
Editor’s note: Parts 1 and 2 are both published in the September/October 2024 issue of Applied Radiology.
Introduction
The last few decades have witnessed a heightened demand for medical imaging in gravid patients, a challenging effort due to medical, ethical, and legal considerations that may arise when imaging both the parent and the fetus. Theoretically, radiologists can use all imaging modalities to evaluate the pregnant patient, but confusion regarding fetus safety often results in unnecessary avoidance of useful diagnostic tests, especially those involving ionizing radiation. Part 1 of this comprehensive review discusses safety guidelines and imaging considerations during pregnancy and lactation, encompassing newer modalities such as contrast-enhanced ultrasound (US), PET/MRI, and pulmonary MR angiography with ferumoxytol. Part 2, presented below, describes specific clinical scenarios (nonobstetric and obstetric) in pregnant patients and the recommended imaging modalities that help answer clinical questions while focusing on fetal and parental safety.
Nonobstetrical Emergency Situations
Trauma
Trauma is the leading nonobstetric cause of death of pregnant patients.1 After trauma, there is an urgent need for quick and accurate imaging of the patient. Diagnostic or interventional radiological studies should not be withheld for concerns of fetal risk of radiation exposure. It should be stressed that the risk of radiation exposure to the fetus is extremely low, as detailed in Part 1. Radiation effects are more deleterious during organogenesis, weeks 2 to 7.
Abdominal US is an invaluable tool in the evaluation of pregnant trauma patients. The focused abdominal ultrasound of trauma (FAST) is the initial imaging method of choice for evaluating pregnant patients involved in trauma, with a reported sensitivity of detection of free fluid in the peritoneal cavity similar to that of the nonpregnant population. It is rapid, safe, and involves no radiation exposure, assessing fetal viability with real-time images of the fetal heart motion and checking for placental injury or acute intraperitonially injuries in the patient.2 The reported sensitivity and specificity values for the detection of intra-abdominal injury in pregnant patients with US range between 61% and 83%, and 94% and 100%, respectively.3
Clinicians must, however, be aware of the limitations of US in pregnant patients with blunt abdominal trauma. When US is negative or inconclusive and the patient is hemodynamically stable, an abdominal CT is warranted given the potential life-threatening injuries that may be missed with US alone. It is advised to limit the area studied, the number of cuts/slices, and the phases of the abdominal CT. Examinations that do not include the gravid uterus (eg, head or chest CT) should be performed without concerns about fetal safety.4, 5 Contrast-enhanced CT is considered the most accurate diagnostic tool available for assessment of solid organ injury in the seriously injured pregnant patient with blunt abdominal trauma.5
MRI in the setting of trauma is indicated only when there is suspicion of spinal cord or neural injuries.6
Suspected Pulmonary Embolism
Pulmonary embolism (PE) occurs with increased frequency in pregnancy owing to the hypercoagulable state of pregnancy and increased venous stasis, with prevalence of venous thromboembolism reported up to 5 times higher in the pregnant populated compared with the nonpregnant state.1 It is the leading cause of mortality in pregnant patients, with mortality rates of undiagnosed PE in pregnancy being up to 15%.1 Treatment with antiocoagulation is associated with morbidity for the patient and the fetus.1, 7 Therefore, it is imperative to exclude the diagnosis of PE in pregnant patients if there is a clinical suspicion. D-dimers value is not reliable in pregnancy and cannot be used to screen for venous thromboembolism given its progressive increase during pregnancy. Hence, diagnostic imaging is the most accurate tool for confirming or excluding PE.
Diagnostic work-up in cases of suspected PE includes an initial evaluation of both lower extremities with compression Doppler US in an attempt to detect deep venous thrombosis.1 When Doppler US is positive for deep venous thrombosis, treatment should be initiated promptly without the need for further imaging.1 If Doppler US is negative, CT pulmonary angiography (CTPA) or scintigraphy (when chest radiograph is negative) are the second-line imaging tests in pregnant patients to exclude PE8 ( Figure 1 ). Ten percent of patients with a high clinical suspicion of PE and negative US results have angiographically proven PE.6 Alternatively, a ventilation/perfusion (V/Q) study can be performed instead of the CTPA.
An 18-week pregnant patient presenting with shortness of breath, hypoxia, and tachycardia. Axial CT pulmonary artery shows saddle pulmonary emboli in the right, main, and left pulmonary arteries (arrows).
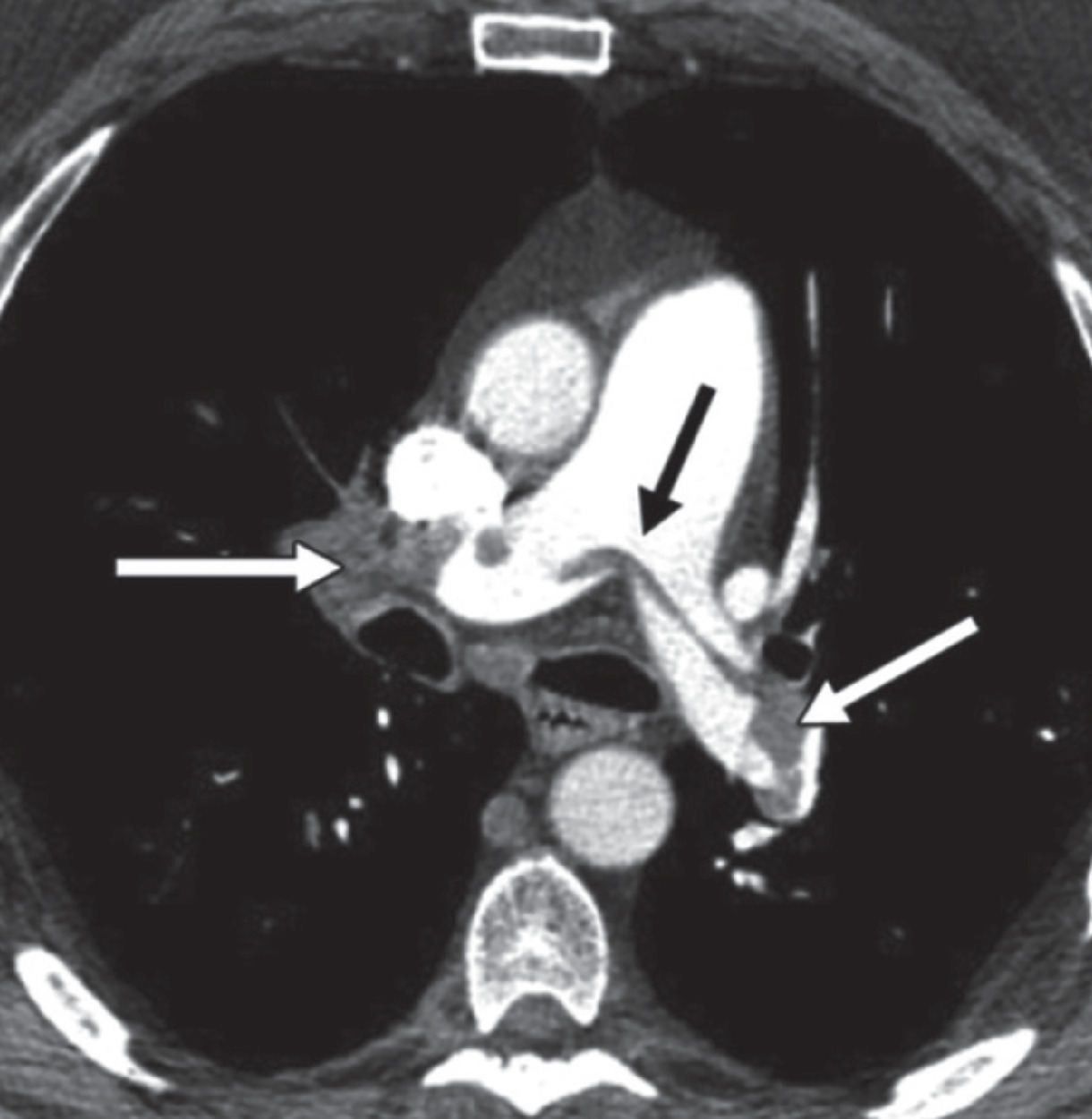
CTPA is preferred in most centers owing to its higher diagnostic accuracy, lower frequency of inadequate studies, and ability to make alternate diagnoses.9 The American College of Radiology (ACR) guidelines rate both imaging studies the same as “Usually Appropriate” and advise that the ventilation portion of the V/Q scan be done only if necessary such as when the perfusion scan is abnormal (ACR Guidelines — Suspected Pulmonary Embolism in Pregnancy revised 2022).10
When investigating suspected PE in pregnancy, the potential radiation exposure risk of the patient and fetus should be discussed with the patient to provide factual data on the risk (extremely low even with CTPA, provided that low-radiation-dose protocols are used) and the consequences of potentially missing a life-threatening diagnosis.
With reduction methods, the estimated conceptus dose is considerably lower than the 100 mGy radiation threshold dose associated with increased risk of organ malformation and childhood cancer. These methods include (1) reducing anatomical coverage of the scan, (2) using iterative reconstructive techniques, (3) reducing the kilovoltage, and (4) reducing the contrast-monitoring component of the CTPA.11 Additional details can be found in Part 1.
CTPA and perfusion scanning appear to have similar negative predictive values and false negative rates when used to investigate patients with suspected PE.11 In the past, CTPA was reported to deliver a radiation dose of as high as 20 mGy per breast. This exceeded the ACR recommendation of 3 mGy or less for standard 2-view mammography.12 In contrast, perfusion scintigraphy delivers 0.11 to 0.31 mGy. However, modern advances in CT technology have significantly reduced the amount of radiation delivered to breast tissue, while also maintaining appropriate image quality. Therefore, using modern imaging techniques, CTPA may expose the maternal breast to median doses as low as 3 to 4 mGy. Such improvements in CTPA dose reduction make maternal cancer risk negligible and encourage the use of CTPA as the first-line modality in pregnant patients with suspected PE.11
Acute Abdominal Pain
Investigating the etiology of acute abdominal pain with or without associated symptoms and signs of nausea, vomiting, and fatigue remains a challenge during pregnancy due to the commonality of such symptoms with the normal physiological pregnant uterus as well as pregnancy-related leukocytosis. As such, physicians are often unwilling to use imaging studies other than sonography, particularly during early gestation, to avoid any harmful effects on the fetus. All the above may result in a delayed diagnosis, leading to increased morbidity and mortality for both the patient and the fetus.
Acute Appendicitis
Acute appendicitis is the most common nonobstetric surgical condition in pregnancy requiring emergency intervention.13 Timely diagnosis of acute appendicitis in the gravid population is important to prevent appendiceal gangrene or rupture, which could lead to premature labor and poor fetal outcome. There is a higher rate of perforation in pregnant patients (43%) compared with the general population (4%-19%), and early diagnosis is essential for optimal treatment.6, 14
ACR Appropriateness Criteria for right lower quadrant pain in the pregnant patient (published in 2018, revised in 2022) recommend abdominal US or abdominal and pelvic MRI without contrast as first-line modalities. These modalities are considered equivalent alternatives (ie, 1 procedure can provide the clinical information).15 Abdominal US is typically used as first-line imaging for suspected appendicitis in pregnant patients at most centers owing to its wide availability and ability to survey the pelvis for alternate diagnoses.1 In instances when US reveals normal or indeterminate findings for appendicitis but high clinical suspicion remains and there is no alternative diagnosis, further imaging with MRI is required to diagnose or exclude abdominal pathology ( Figure 2 ).
Early appendicitis in presenting with right lower quadrant pain at 18 weeks’ gestation. US did not identify the appendix. Coronal T2-weighted single-shot fast spin echo of the right midabdominal shows the high T2 signal within the appendiceal lumen; the appendix is curved medially and is shifted superiorly outside the iliac fossa, along with the base of the cecum (star) due to the displacement from the gravid uterus. A rounded low-signal intensity in the proximity of the appendix (arrow) represents an appendicolith. Mild peri-appendiceal fat stranding is seen as a high-intensity signal in the adjacent fat. Acute appendicitis was confirmed at surgery.
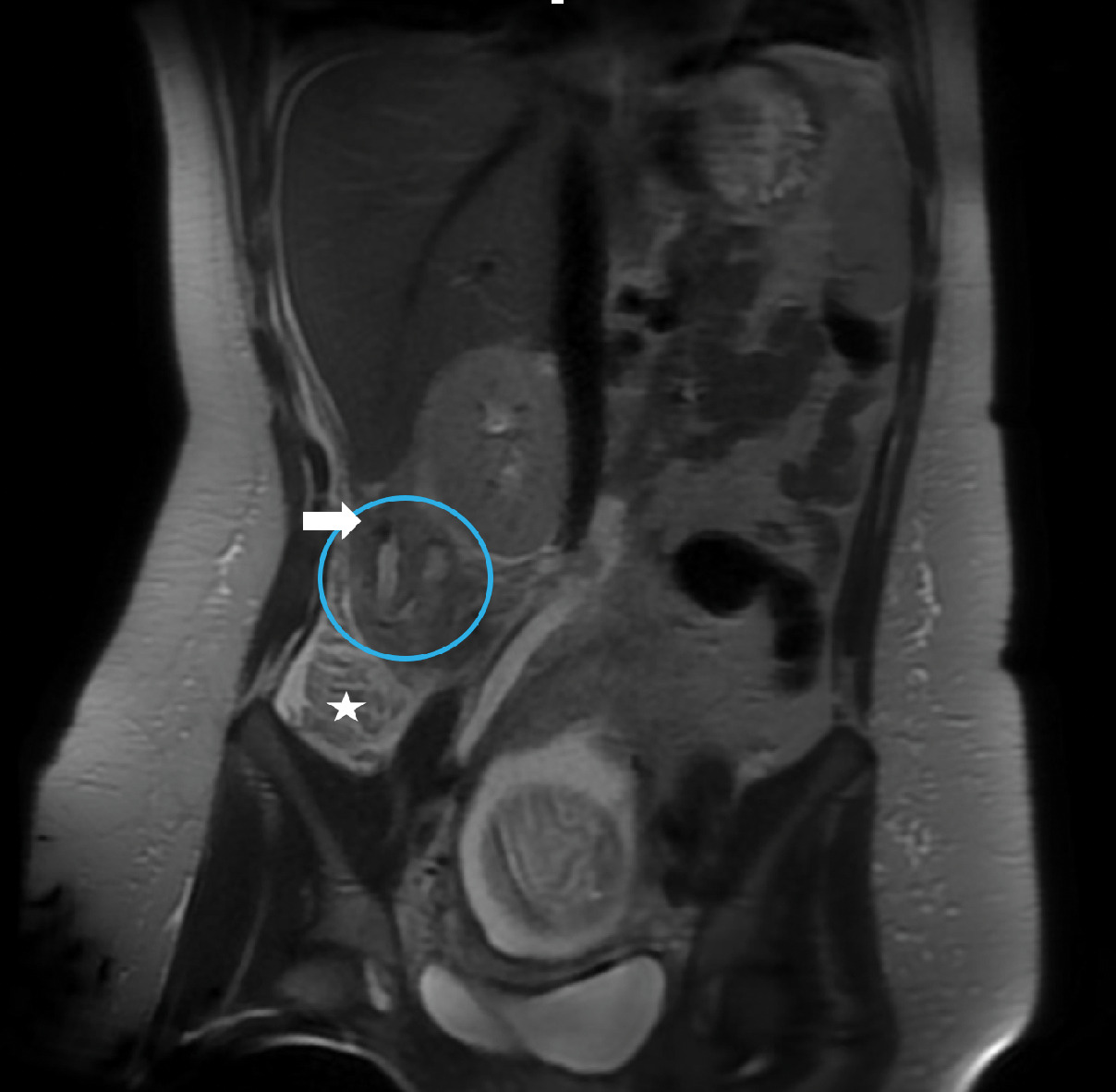
If, for any reason, MRI cannot be performed (contraindicated or not available), abdomino-pelvic CT with oral and intravenous (if necessary) administration of contrast material can be used.4, 14 Some authors propose an algorithm including US and low-dose CT (LDCT, < 2.5 mSv) as first and second imaging steps, respectively, to assess pregnant patients with a suspicion of appendicitis; LDCT seems to be highly accurate for diagnosing appendicitis and can be used when MRI is not immediately available.16
Urolithiasis
Urolithiasis is the most common painful nonobstetric condition and nonobstetric reason for hospitalization in pregnant patients. If it remains undiagnosed or untreated, urolithiasis may result in pyelonephritis or even premature labor induced by renal colic.17
According to the ACR Appropriateness Criteria, US is the first-line imaging in pregnant patients with flank pain. It has reasonably good sensitivity in detecting obstructive renal stones (34%-95.2%) and no potential ionizing radiation exposure to the patient or fetus (rating of 8, or “Usually Appropriate”). US can accurately diagnose pelvicaliectasis and ureterectasis; however, physiological dilation of the collecting system from compression of the mid ureter between the gravid uterus and the sacral promontory is common in this population.14
When US is inconclusive or fails to establish a diagnosis and there are continued signs or symptoms despite medical treatment, ACR guidelines recommend either noncontrast LDCT (< 3 ms) or MR urography as second-line tests. The noncontrast LDCT (rating of 6, or “May be Appropriate”) is the radiologist’s preferred modality given its high accuracy (sensitivity of 97% and specificity of 95%) compared with MR urography (rating of 5, or “May be Appropriate”), particularly in the second and third trimesters when MRI is less accurate for identifying suspected stones. MRI is highly accurate in depicting hydronephrosis and perinephric edema. Evaluation of the urinary tract in the pregnant patient can be accomplished with T2-weighted images (ie, HASTE sequence).18, 19
Acute Cholecystitis
Biliary tract obstruction is a relatively common disorder in pregnant women and the second most common nonobstetric emergency during pregnancy. Pregnant patients are at an increased risk of biliary obstruction secondary to decreased gallbladder contractility, increased cholesterol synthesis, and increased bile stasis during pregnancy, which result in stone formation in 2% to 4% of pregnant patients.14 Potential complications of untreated acute cholecystitis include obstructive jaundice, gallstone pancreatitis, peritonitis, and potential fetal loss.1, 14
US of the right upper quadrant is the initial imaging modality of choice for evaluating the gallbladder and the biliary tree14 ; however, when acute cholecystitis, choledocholithiasis, or pancreatitis are suspected and US findings are normal or inconclusive, MR cholangiopancreatography (MRCP) is the second-line imaging modality.14, 20 MRCP is noninvasive, and gadolinium contrast administration is not necessary; T2-weighed sequences depict the hepatobiliary tree and pancreatic duct in excellent detail, helping to identify biliary or pancreatic pathology. MRCP is superior to US, depicting comprehensive visualization of the biliary system in great detail, as well as investigating other causes of acute abdominal pain with the lack of ionizing radiation. It allows the radiologist to assess with greater confidence the exact location of stone impaction in the cystic duct and/or the common bile duct, and differentiate, based on signal intensity of the gallbladder wall, between acute and chronic cholecystitis.20
The use of MRCP in pregnant patients with biliary ductal dilatation by US has been shown to obviate further exploration with endoscopic retrograde cholangiopancreatography (ERCP). Although studies have shown that ERCP is safe in pregnant women, its use should be limited to instances when therapeutic intervention is necessary.20
Obstetrical Nonfetal Emergency Situations
Ovarian Torsion
Ovarian torsion is more frequent in pregnant patients and more common in the first trimester. It occurs most commonly secondary to large adnexal masses, which have been reported in up to 1% in pregnancy, although most are functional cysts and regress by week 16 of pregnancy.21 Persistent masses present a risk for ovarian torsion, particularly when the mass is over 4 cm, with an estimated incidence of 1 in 1800 pregnancies, most commonly occurring in the early gestational period.22 Diagnosis of ovarian torsion is primarily based on clinical presentation of acute pelvic pain. US is complementary for establishing a diagnosis, and MRI is reserved in cases of undiagnostic or equivocal findings on US.6
Degenerating Leiomyoma
Degenerating leiomyomas are common in pregnancy, with an incidence of up to 10.7%. The increased levels of estrogen and the rapid uterine growth cause leiomyomas to grow rapidly and outgrow their blood supply, resulting in infarction and hemorrhagic degeneration (red degeneration). This is a painful condition, which may present as an acute abdomen and may cause premature rupture of membranes.22 Typically, US is the first-line modality for evaluating a degenerated leiomyoma; if needed, MRI may provide more detailed anatomic information and can specify the location, size, and degree of degeneration. Furthermore, specific MRI characteristics allow for radiological differentiation between leiomyoma (often managed conservatively, or with minimally invasive treatment) and the rare, but aggressive, leiomyosarcoma.23
Placental Disorders
Placental abruption is the premature separation of the placenta from the uterine myometrium after gestational week 20 and may occur in up to 1.3% of pregnancies. Patients typically present with abdominal pain and severe vaginal bleeding. Early detection is paramount as there is an associated fetal mortality and morbidity rate of 10% to 25%.22 US is the first-line tool to confirm the clinical diagnosis. MRI should be considered when US is negative but high clinical suspicion for abruption remains; this is particularly important in placental abruption with separation greater than 50%, which is associated with a higher risk of fetal death.
Placental accreta spectrum disorder (PASD) is a potentially fatal condition of pregnancy, especially if it remains undiagnosed, with an increasing incidence in those with advanced age, prior Caesarean section, multiparity, prior placenta previa, or uterine curettage.22
US is the initial modality for screening and diagnosing PASD. However, to overcome the unfavorable anatomical placental locations and physiological limitations of high body mass index, an MRI can be performed in addition to US to improve results of prenatal imaging. MRI has been shown to improve detection of abnormal placentation into the myometrium and uterine serosa, and increase visualization in posteriorly located placentas when US is inconclusive ( Figure 3 ). In addition, due to MRI’s more comprehensive full pelvic coverage, radiologists can use the modality to more confidently diagnose cases of extrauterine implantation of placenta percreta.24 A 2022 study determined that the diagnostic performance of 1.5T and 3.0T MRI are equivalent in diagnosing PASD.25
Third-trimester gestation with concern for placenta accreta on initial diagnostic US (not shown). T2-weighted HASTE sequences in axial (A) and 2 coronal planes; anterior (B) and a more posterior coronal slice (C), the latter showing complete placenta previa (star). Multifocal areas of myometrial thinning along the mid anterior and left anterior uterus (arrows), with adjacent areas of focal placental bulge were concerning for placenta increta. Placenta accreta was found in pathology (case courtesy of Dr Aman Khurana).
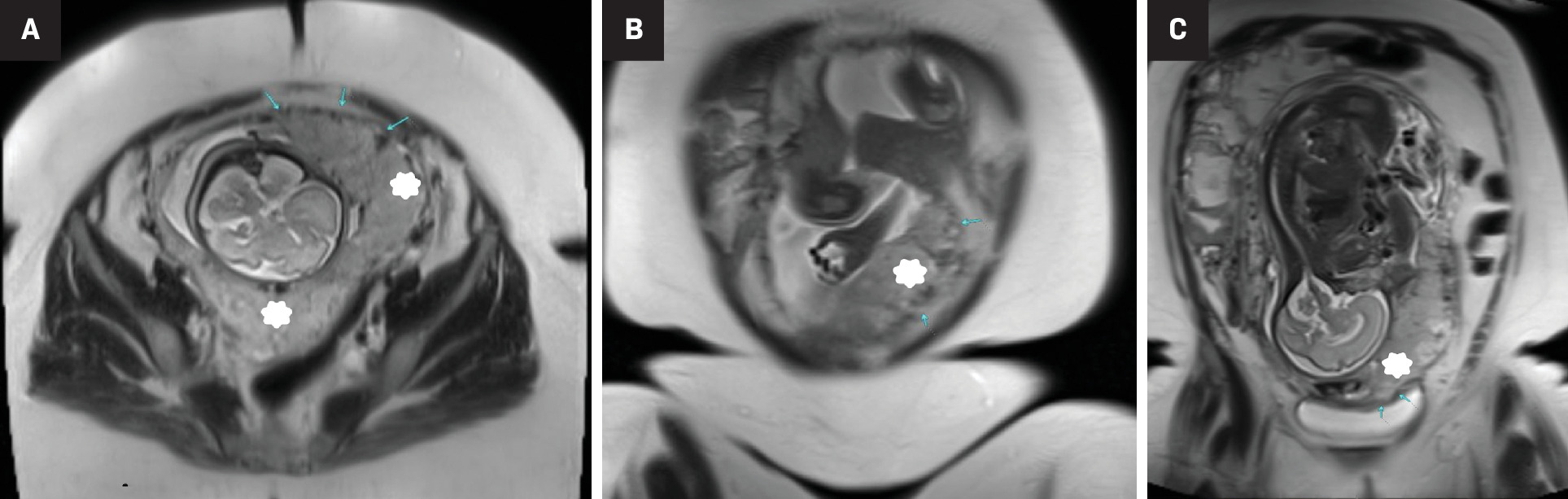
Prognosis can be substantially improved by having a PASD diagnosis as it can prompt management strategies to best meet parental expectations.24
Cancer Diagnosis and Staging
Cancer in pregnancy is rare; in a recent 20-year international cohort of 1170 patients with pregnancy-associated cancers, breast cancer was the most common malignant disease diagnosed in 39%, while gynecological tumors and hematological malignancies accounted for 20% and 16%, respectively.26
US is the first-line study for evaluating a palpable breast mass in the pregnant or lactating patient, with a reported sensitivity and negative predictive value of 100% for pregnancy-associated breast carcinoma.27 For suspicious masses, biopsy should be performed immediately. Mammography and noncontrast MRI of pregnant patients are safe and complementary imaging modalities, particularly in patients with a highly suspicious mass on physical examination or US, and in patients newly diagnosed with breast cancer.
The amount of radiation associated with a mammogram is low and limited to the breasts. The ACR Appropriateness Criteria requires the average glandular dose (as it applies to full-field digital and screen mammography) delivered by a single craniocaudal view of 4.2-cm-thick, compressed breast of 50% glandular and 50% adipose tissue to not exceed 3.0 mGy, although it is generally much lower.28 In a recent retrospective study of 374 patients comparing radiation dose delivered by digital breast tomosynthesis (DBT) vs full-field digital mammography (FFDM), it was found that the mean glandular doses for both breasts were lower in DBT (left 1.74, right 2.1) compared with FFDM (left 2.85, right 2.74).29
The radiation dose to tissues other than the breast from standard bilateral 2-view mammography is extremely low. The dose to the first-trimester fetus is negligible (< 10-5 mGy),30 supporting previously reported estimated dose to the uterus of less than 0.03 mGy.6
A lead shield placed over the lower part of the abdomen to protect the gravid uterus from scatter radiation was historically reported to reduce the dose to the uterus.30 However, the recently updated ACR-SPR Practice Parameter for Imaging Pregnant or Potentially Pregnant Patients with Ionizing Radiation (revised 2023), recommend against abdominal shielding, citing that higher scatter radiation from the lead shield increases fetal exposure.31
Gynecological malignancies, especially cervical and ovarian cancer, are commonly diagnosed during pregnancy. Imaging is an important part of the diagnosis, staging, and follow-up of pregnancy-associated gynecologic tumors, with both US and MRI being the preferred modalities. MRI has many advantages for staging cancer during pregnancy as it provides a larger imaging field of view, multiplanar capability, better reproducibility among readers, and excellent pelvic soft-tissue contrast. MRI is particularly useful in cervical cancer for evaluating tumor size, nodal involvement, and extra pelvic disease ( Figure 4 ).
Third-trimester gestation. Sagittal T2 HASTE sequence (A) shows a gravid uterus with an anterior normal placenta and abnormal isointense irregular cervical mass, digitally magnified in (B) (circle). The true/oblique axial T2 HASTE images along the cervical length (C1 top and C2 bottom) show disruption of the hypointense cervical ring at the 4-8 o’clock position (arrows), criterion for >stage IIb cervical cancer. Coronal T2-weighted image (D) shows right pelvic heterogenous and enlarged lymph node (circle), suggesting regional pelvic metastasis (case courtesy of Dr Aman Khurana).
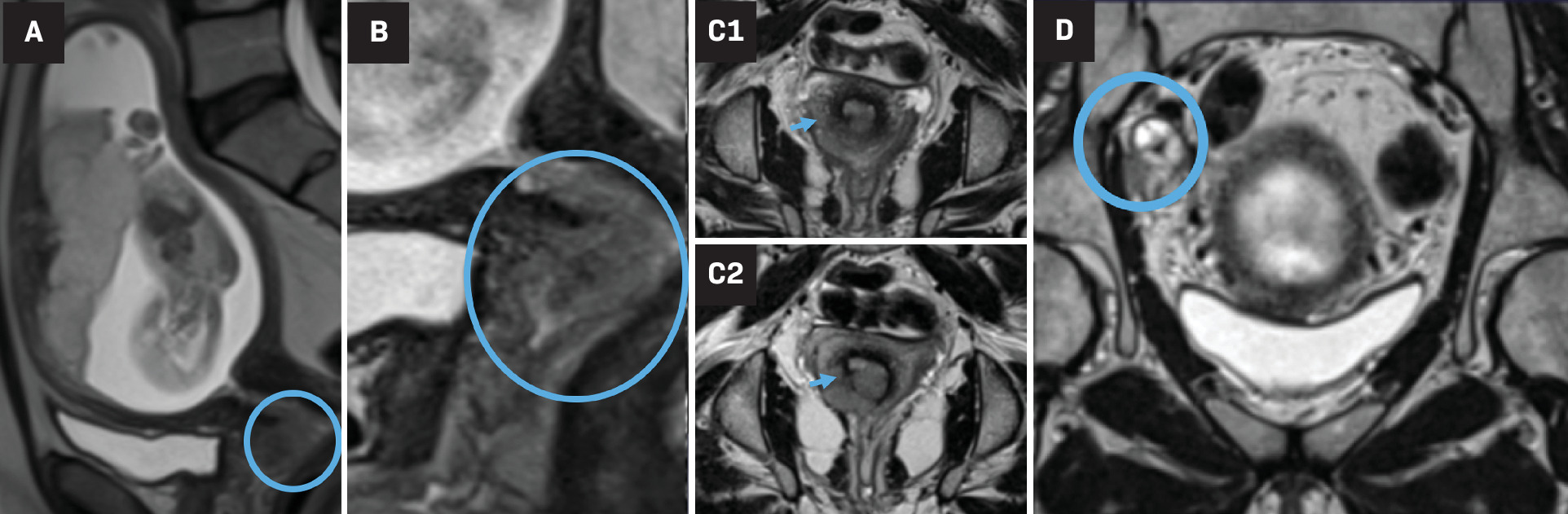
Ovarian tumors are typically initially diagnosed with sonography; MRI can be valuable in cases of indeterminate US findings as well as for staging.32
A single-center pilot study in Belgium published in 2018 suggested the utility of whole-body diffusion-weighted imaging MRI (WB-DWI/MRI) using the functional tissue properties for the oncological staging of pregnant patients without the need for a contrast agent. The study, which looked prospectively at 20 pregnant patients comparing WB-DWI/MRI with conventional imaging, concluded that WB-DWI/MRI was helpful in assessing oncological pregnant patients and was more accurate than conventional imaging during pregnancy.33 With recent technological innovations through thin-slicing acquisition and increased special resolution, this technique provides promise for tumor screening and staging.
Conclusion
Imaging has an increasing role in the diagnosis and management of parental pathology during gestation. Knowledge of current imaging recommendations and safety guidelines for the pregnant population may help referring physicians and radiologists select the most appropriate modality to image the expectant patient. Potentially fatal conditions such as PE, trauma, PASD, and pregnancy-associated cancers can be identified early and accurately with available imaging methods, thus improving outcomes. MRI is a useful tool to evaluate numerous obstetric and nonobstetric conditions in pregnancy. In addition to being radiation-free, MRI offers several significant diagnostic advantages over CT and US.
References
Citation
Clark A, Wang X, Khouli RE, Szabunio M.Quality and Safety in Medical Imaging During Pregnancy and Lactation — Part 2. Appl Radiol. 2024; (5):16 - 22.
doi:10.37549/AR-D-24-0027
October 1, 2024