Pulmonary Hypertrophic Osteoarthropathy
Images

Case Summary
A adoloscent with history of severe food allergies requiring oral immunotherapy, iron deficiency anemia, and eczema presented to the rheumatology clinic with a two-month history of lower extremity joint swelling and extreme fatigue following minimal exertion. The physical exam was notable for knee and ankle joint effusions with tenderness to palpation. Clubbing of the fingers and toes was present bilaterally. Laboratory evaluation revealed elevated erythrocyte sedimentation rate, C-reactive protein, and serum IgA. Notable negative labs included complete blood count, comprehensive metabolic panel, antinuclear antibody, Lyme disease, and rheumatoid factor.
Imaging Findings
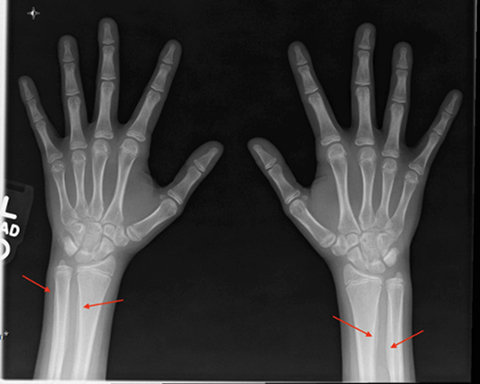
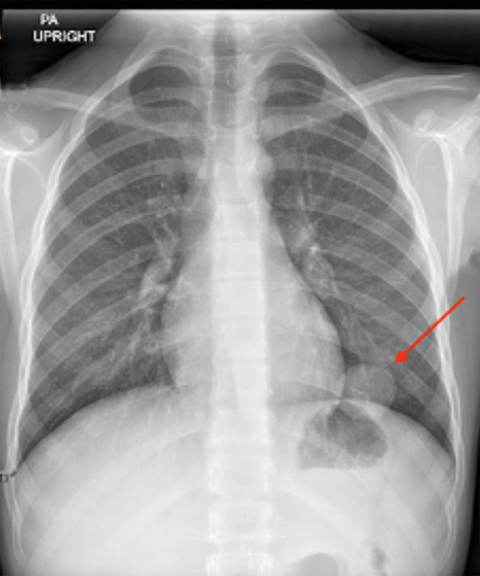
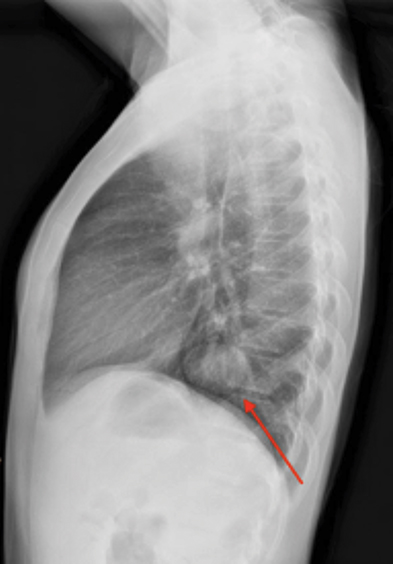
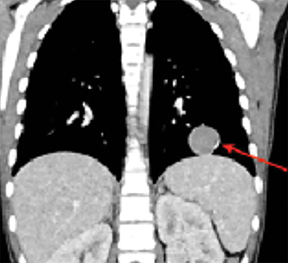
X-ray of the hands and wrists (Figure 1) showed smooth periosteal thickening of the distal radius and ulna bilaterally without an underlying bone lesion. A chest radiograph (Figure 2) revealed a well-circumscribed 3.7 cm ovoid mass in the left lower lobe.
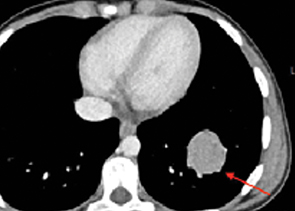
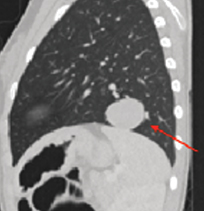
Further characterization with contrast-enhanced chest CT (Figure 3) demonstrated a well-demarcated, solitary homogeneous soft tissue density mass in the left lower lobe abutting the left hemidiaphragm. CT of the head, abdomen, and pelvis showed no evidence of extrapulmonary disease.
Subsequent left lower lobe wedge resection was performed, and inflammatory myofibroblastic tumor was confirmed by histologic analysis. The patient experienced nearly complete resolution of her lower extremity arthritis and fatigue.
Diagnosis
Pulmonary hypertrophic osteoarthropathy secondary to inflammatory myofibroblastic tumor (IMT).
Discussion
Hypertrophic osteoarthropathy (HOA) is characterized by the clinical triad of digital clubbing, periosteal reaction of the long bones, and polyarthritis.1 HOA can be classified as primary and secondary. Primary HOA (also known as pachydermoperiostosis) is a rare autosomal dominant genetic disorder accounting for 5% of cases. Secondary HOA results from extraskeletal disease and comprises 95% of cases.
The majority of secondary HOA cases occur in adults and are commonly attributable to pulmonary malignancy, most notably non-small cell lung carcinoma.1 Secondary HOA is rare in children and is more commonly associated with chronic disease processes such as cystic fibrosis, congenital heart disease, endocarditis, pulmonary fibrosis, or inflammatory bowel disease.2 Malignancy accounts for only 12% of secondary HOA in the pediatric population, with nasopharyngeal carcinoma, Hodgkin lymphoma, osteosarcoma, and pulmonary squamous cell carcinoma all having been reported.2,3 Our patient demonstrated HOA secondary to a pulmonary IMT, a rare pediatric tumor with only 150 reported cases in the United States.4 To our knowledge, only three other cases of HOA secondary to IMT have been reported.5
The pathophysiology of HOA remains uncertain. However, multiple proposed mechanisms suggest elevation of platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) as inciting factors for development of clubbing and periosteal reaction.6 Some hypotheses suggest hypoxia or inflammation-induced elevation of systemic PDGF and VEGF (as in cystic fibrosis, endocarditis, or inflammatory bowel disease). Another implicates right-to-left shunts (as in congenital heart malformation or intratumoral shunts) causing microthrombi to lodge in distal extremity arterioles, resulting in local elevation of PDGF and VEGF.6
One diagnostic pitfall of HOA is the nonspecific presentation of the disease. Most patients do not present with the classic triad of clubbing, periostitis, and arthritis. Often, joint effusions are the only presenting symptom.7 On physical exam, large joint effusions with associated pain, tenderness, and swelling is common. Less commonly, tenderness to palpation of the long bones, secondary to periostitis, and nail clubbing may be present. Laboratory findings are also nonspecific, and arthrocentesis generally results in normal leukocyte count.8
Owing to its rarity in children, HOA provides a particular diagnostic challenge as the differential for bone pain and arthritis is very broad and includes trauma, septic arthritis, reactive arthritis, growing pains, juvenile idiopathic arthritis, Lyme disease, transient synovitis, and primary bone malignancies. Thus, imaging studies are crucial in the evaluation of HOA.
Given the primary presenting symptom of HOA is often arthritis, initial imaging is often centered around the affected joints. Radiography typically demonstrates effusion with absence of joint space narrowing, periarticular osteopenia, osteophyte formation, and erosions.9
The key radiographic feature of HOA is symmetric, smooth, continuous periosteal reaction of the tubular bones (most commonly the tibia, fibula, radius, and ulna).9 The periostitis appears as a monolayer of periosteal new bone formation around the tubular bone diaphysis with preservation of the bone’s shape. With disease progression, multilayered or laminated periostitis may be seen with involvement of the metaphysis and epiphysis.8
Bone scintigraphy may show the tubular bone periosteal reaction. Blood pool images reveal periosteal hyperemia, while delayed images show increased linear periosteal tracer uptake running parallel to the normal cortical bone (“double line” sign).8
While other bony processes may produce periostitis, the presence of bilateral smooth periosteal reaction without an underlying lesion should invoke suspicion for HOA and prompt further evaluation with chest and abdominal imaging.
The mainstay of therapy for secondary HOA is treatment of the underlying disease process. In our case, the patient experienced near-complete symptomatic resolution following resection of the primary pulmonary inflammatory myofibroblastic tumor. Multiple cases have been reported of symptomatic and radiographic resolution following treatment of the underlying primary etiologies.10 These include resection of primary lung lesions, correction of cyanotic heart disease, antibiotic treatment for recurrent lung infections, lung transplant in cystic fibrosis patients, and liver transplants in end-stage cirrhosis. When primary treatment is not feasible, symptomatic improvement has been reported with NSAIDs, bisphosphonates, and octreotide. Newer therapies are targeting VEGF inhibition with bevacizumab, an anti-VEGF antibody.10
Conclusion
Hypertrophic osteoarthropathy is a rare pathologic process in children that provides a diagnostic challenge owing to its nonspecific clinical presentation. However, it is important for the radiologist to consider the diagnosis in a patient with symmetric smooth periosteal reaction of the tubular bones. If present, further assessment with chest and abdominal imaging is warranted to evaluate for an underlying lesion or etiology.
References
- Ito T, Goto K, Yoh K, et al. Hypertrophic pulmonary osteoarthropathy as a paraneoplastic manifestation of lung cancer. J Thorac Oncol. 2010;5(7):976-980. https://doi.org/10.1097/JTO.0b013e3181dc1f3c
- Utine EG, Yalçin B, Karnak I, et al. Childhood intrathoracic Hodgkin lymphoma with hypertrophic pulmonary osteoarthropathy: a case report and review of the litera
- ture. Eur J Pediatr. 2008;167(4):419-423. https://doi-org.ezproxy.lib.usf.edu/10.1007/s00431-007-0522-z
- Cao C, Otjen JP, Shenoi S. Secondary hypertrophic osteoarthropathy associated with pediatric primary lung squamous cell carcinoma. J Clin Rheumatol. 2020;26(1):10-11. doi: 10.1097/RHU.0000000000000722
- Greenhill M, Aria D, Schaefer CS, Kaye R, Jorgensen SA, Abruzzo T, Towbin A, Towbin R. Inflammatory Myofibroblastic Tumor. Appl Radiol. 2020;49(1):56D-56F.
- G Pichler, E Eber, G Thalhammer, W Muntean, MS Zach. Arthralgia and digital clubbing in a child: hypertrophic osteoarthropathy with inflammatory pseudotumour of the lung, Scandinavian Journal of Rheumatology. 2004;33(3): 189-191. doi: 10.1080/03009740310004702
- Callemeyn J, Van Haecke P, Peetermans WE, Blockmans D. Clubbing and hypertrophic osteoarthropathy: insights in diagnosis, pathophysiology, and clinical significance. Acta Clin Belg. 2016;71(3):123-130. doi: 10.1080/17843286.2016.1152672
- Izumi M, Takayama K, Yabuuchi H, Abe K, Nakanishi Y. Incidence of hypertrophic pulmonary osteoarthropathy associated with primary lung cancer: Incidence of HPO in lung cancer patients. Respirology. 2010;15(5):809-812. doiI: 10.1111/j.1440-1843.2010.01769
- Taljanovic MS, Omar IM, Hoover KB, Chadaz TS, eds. Musculoskeletal Imaging Volume 1: Trauma, Arthritis, and Tumor and Tumor-like Conditions. Oxford University Press; 2019.
- Yap FY, Skalski MR, Patel DB, et al. Hypertrophic osteoarthropathy: Clinical and imaging features. Radiographics. 2017;37(1):157-195. doi: 10.1111/j.1440-1843.2010.01769
- Nguyen S, Hojjati M. Review of current therapies for secondary hypertrophic pulmonary osteoarthropathy. Clin Rheumatol. 2011;30(1):7-13. doi: 10.1007/s10067-010-1563-7
References
Citation
TB F, AJ T, RB T, C S, JN K.Pulmonary Hypertrophic Osteoarthropathy. Appl Radiol. 2021; (5):45-47.
September 10, 2021