Problem Solving with Breast MRI
Images



Magnetic resonance imaging plays a well-established role in breast cancer screening for patients at increased risk for the disease, as well as in evaluating patients with new breast cancer diagnoses. However, breast MRI’s role in problem solving continues to evolve. The modality is appropriately indicated in cases where MRI findings have the potential to affect clinical management and cannot be answered with either mammography or sonography. Here we review accepted clinical scenarios for problem solving with breast MRI.
Monitoring Lumpectomy Sites
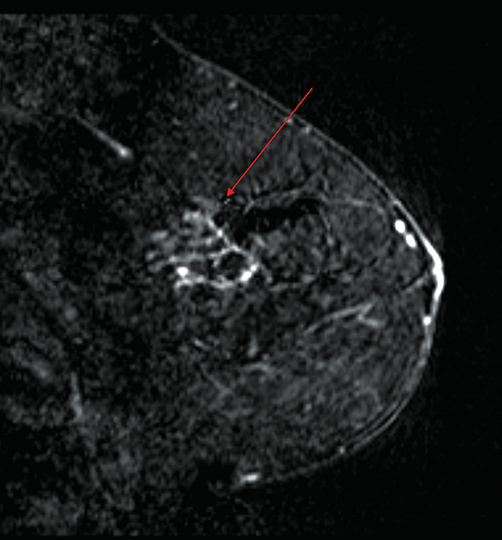
Mammography and clinical examination are the standard of care with respect to screening patients for recurrent disease at lumpectomy sites. However, breast MRI can be a particularly effective diagnostic tool in certain circumstances. For example, post-treatment changes can mask or mimic recurrent disease at lumpectomy sites. Leveraging morphological features and signal intensity curves, MRI boasts high sensitivity, specificity, and accuracy in differentiating post-treatment physiological changes from recurrent disease.1 A literature review by Preda, et al, found MRI able to detect recurrent disease with a sensitivity > 90% and specificity of 85-100%.1 MRI can also be particularly useful in assessing for recurrent disease in patients who have undergone radiation therapy to a lumpectomy site in the posterior third of the breast who may be unable to undergo mammography because of scarring and tissue retraction (Figure 1).
Lumpectomy with Positive Margins
Breast conservation surgery is the treatment of choice in early-stage breast cancer. After surgical excision, negative margins reduce the risk of residual and recurrent local disease. Positive margins have been reported in 15-37% of patients undergoing lumpectomy, resulting in the need for additional re-excision or mastectomy.2 Accurately determining gross residual disease, when present, is imperative in surgical planning, especially in the absence of preoperative MRI.
Breast MRI’s role in the postoperative setting is to detect gross residual disease, as microscopic disease has already been confirmed pathologically. Krammer, et al, reported a sensitivity of 73% for MRI when used to detect residual disease in patients with positive margins after initial surgery.3 However, sensitivity increases to 95% when the size threshold for detecting residual disease is ≥ 5 mm, supporting the modality’s reliability in detecting gross residual disease.3
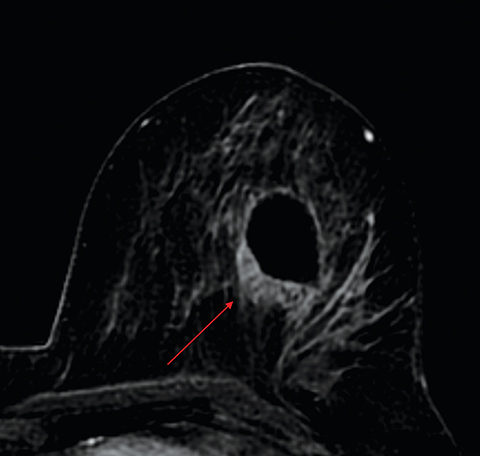
Difficulty in differentiating postsurgical enhancing granulation tissue at the lumpectomy site from residual disease limits the accuracy of breast MRI. A lumpectomy site with no gross residual disease has a thin rim of peripheral enhancement (≤2 mm) without nodularity or focal mass. Gross residual disease on postoperative MRI can present as an enhancing mass (Figure 2) or as non-mass-like enhancement (Figure 3), depending on whether the residual disease is invasive or in situ. Acceptable timing of postoperative MRI has been debated and ranges from 28 days to 10 months following surgery.4 Increasing the lumpectomy-to-MRI interval may improve sensitivity and specificity, but delaying treatment is neither ideal nor necessary.4 Remembering that breast MRI’s role in this setting is to differentiate, based on the extent of residual disease, those patients who need mastectomy from those who require repeat lumpectomy can ease anxiety when interpreting these exams. At our institution, patients are imaged as soon as they are able to lie flat and tolerate the scan, allowing for prompt surgical management.
Response to Neoadjuvant Chemotherapy
Traditionally, neoadjuvant chemotherapy is used to treat locally advanced disease and inflammatory breast cancer. However, it is increasingly being used as part of the treatment of earlier stage disease.5 Neoadjuvant chemotherapy has many benefits, including: reducing tumor size which leads to less extensive breast surgery and better cosmetic outcomes, converting node-positive disease to node-negative thereby reducing the need for axillary surgery, evaluating the efficacy of systemic therapy, and bringing about a pathologically complete response to therapy which correlates with improved disease-free survival.5
Currently, there are no clinical practice guidelines identifying the most accurate method to assess for tumor response to therapy. Physical exam, mammography, and ultrasound are traditionally used, but several meta-analyses have demonstrated that MRI more accurately predicts residual tumor size than do the traditional approaches.5,6 One prospective study using MRI to measure pathologic tumor size after surgical excision demonstrated a response agreement of 71%, compared with clinical examination (19%), mammography (26%), and sonography (35%).6 MRI overestimated residual disease in 6% of patients and underestimated residual disease in 23% of patients.6
Tumor subtype and chemotherapy affect MRI’s ability to accurately estimate the presence of residual disease in the neoadjuvant setting.5 Underestimation is more common in the setting of disease fragmentation or in cases of treatment with taxanes or antiangiogenic drugs.5 The antiangiogenic property inhibits blood flow and gadolinium contrast into any residual tumor. Overestimation is often related to reactive inflammation and fibrosis at the tumor site.5 Additionally, patients can be categorized as having a “complete response” by pathologic response criteria with ductal carcinoma in situ (DCIS). This can limit MRI’s accuracy in assessing for pathologic complete response, as the noninvasive component of disease may still be seen and reported as non-mass enhancement.
Optimal breast MRI timing in the neoadjuvant setting depends on treatment regimen. At our institution, MRI is done at baseline, 2 weeks after the first cycle of chemotherapy to evaluate for early response, and just before surgery. If both an anthracycline-based chemotherapy and a taxane are being used, MRI is obtained after administration of the anthracycline and before the taxane (between regimens). Response range is described as complete (no enhancement or mass to suggest disease, Figure 4), partial (30% decrease in the longest diameter), stable (no partial or progressive disease), or progressive (≥20% increase in the longest diameter, Figure 5).7
Axillary Metastases and Occult Primary Tumor
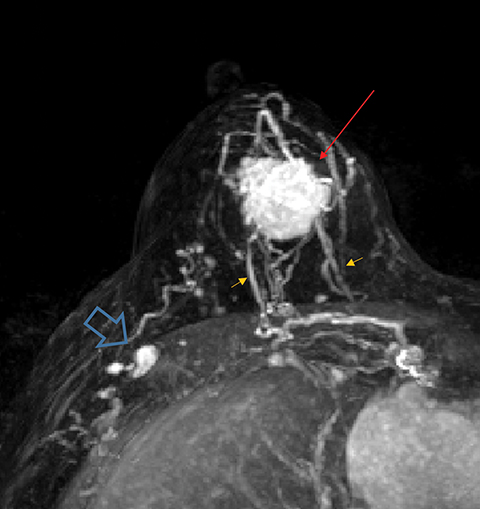
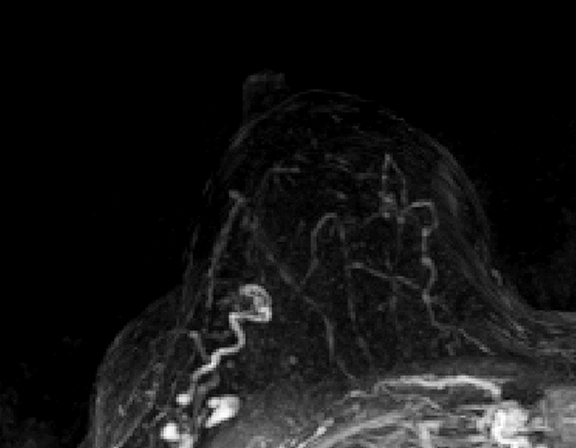
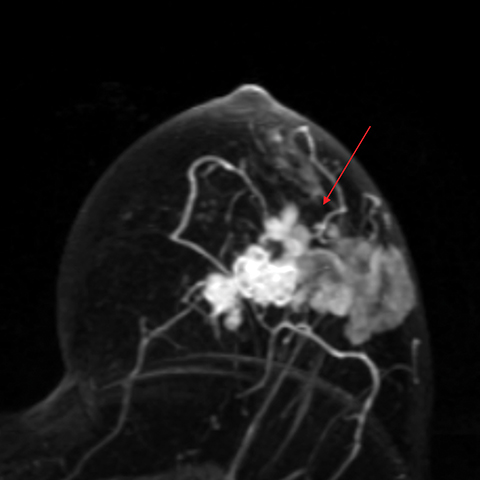
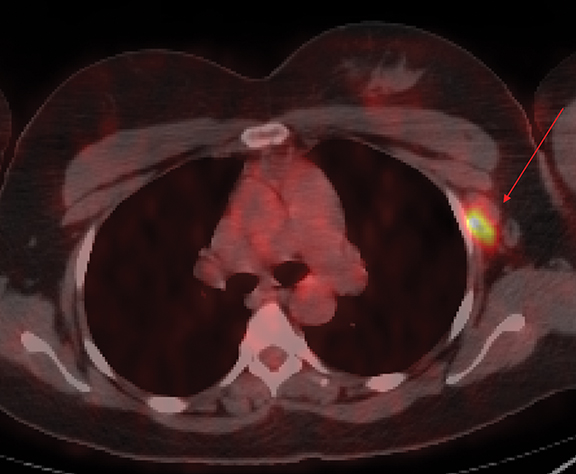
Occult primary breast cancer presents with axillary lymphadenopathy or metastatic disease without clinical, mammographic, or sonographic findings. This is uncommon, representing only 0.3-0.8% of such cancers.8 When occult primary breast cancer does occur, it can present a diagnostic and therapeutic challenge. These patients are first treated with ipsilateral mastectomy and axillary lymph node dissection, the standard of care.9 Detection of these cancers with MRI permits assessment of disease extent, which may potentially increase treatment options, including breast-conservation therapy. Biopsy with histologic evaluation may also be used to guide potential neoadjuvant therapy prior to surgery (Figure 6).
Bresser, et al, reviewed 8 retrospective studies that define occult breast cancer as isolated metastatic axillary lymphadenopathy with negative clinical and mammographic examinations (ultrasound not included). MRI was able to detect a suspicious finding in 36-86% (mean of 72%) of patients; 85-100% of these resulted in diagnosis of malignant breast cancer. Pooled data demonstrates that MRI has sensitivity of 90% and specificity of 31% in detecting occult primary breast cancer. Five of the 8 studies utilized MRI-guided ultrasound localization, finding that 80% of the MRI detected-lesions could be localized with ultrasound. Ultimately, routine use of breast MRI in occult breast cancer may result in breast conservation therapy in as much as one-third of cases.10
Evaluation of Nipple Discharge
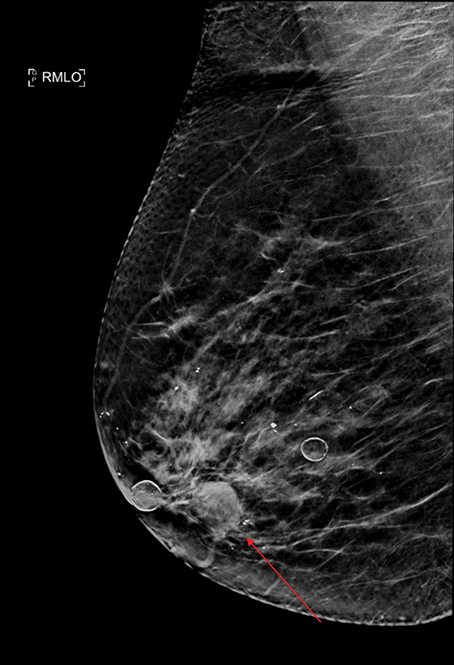
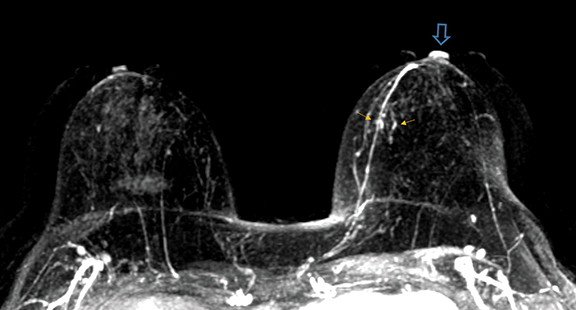
Spontaneous nipple discharge is most commonly due to benign etiologies, including papilloma or duct ectasia. However, a thorough workup should be performed to exclude underlying DCIS or IDC.11 A detailed history is needed to determine whether the discharge is spontaneous or nonspontaneous, the latter being less concerning. Historically, the standard of care for patients with spontaneous discharge and a normal mammogram and ultrasound is a central duct excision. However, this poses significant risk that the abnormal area may not be excised, especially with abnormalities located posteriorly (Figure 7).12
A physical exam performed after mammography may determine if the discharge is reproducible and from a single duct. Ultrasound is helpful in the presence of a dilated duct with associated intraductal mass. If these are not present, MRI evaluation is warranted.
As it has high sensitivity and specificity for diagnosing breast cancer, MRI is useful for detecting malignant etiologies of nipple discharge. In a study of MRI evaluation of pathologic nipple discharge, the modality detected all 11 cases of malignancy, 7 of which were occult on mammography and sonography.12
Paget Disease
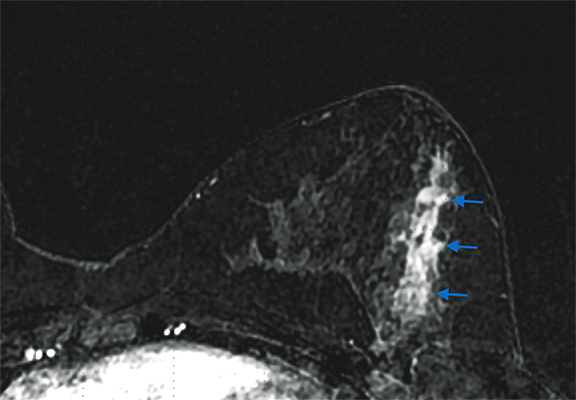
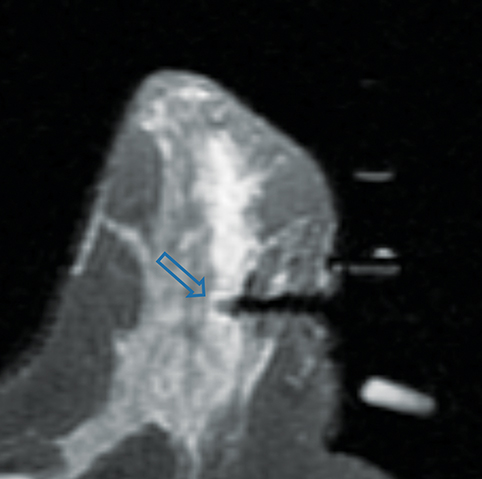
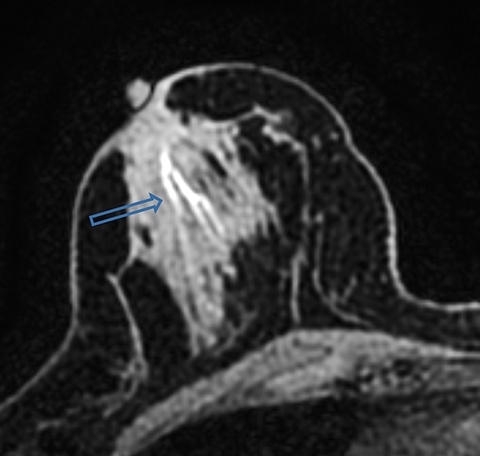
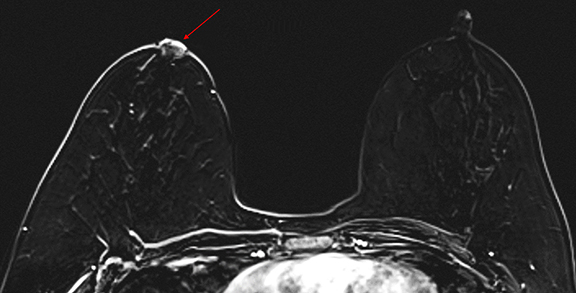
Paget disease of the breast is uncommon, accounting for 1-3% of all breast carcinomas.13 It is defined as infiltration of the nipple epidermis by adenocarcinoma cells.13 Paget disease is often detected clinically; classic symptoms include redness and itching of the nipple areolar complex, with progression to scaling, ulceration, and erosion. Studies report underlying malignancy in 90% of Paget disease cases, but up to 50% of patients present with a negative mammogram.13 Accurate detection and assessment of the extent of disease are critical in determining appropriate therapy and surgical management.
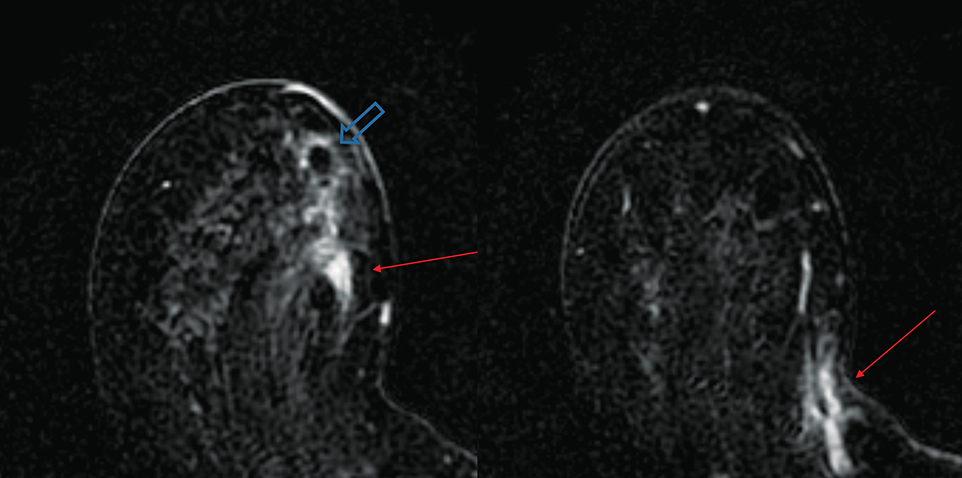
Several studies have demonstrated the utility of MRI in detecting underlying cancers in patients with Paget disease. Not only can MRI detect mammographically occult cancers, but it is also more sensitive than mammography in evaluating extent of disease.13 In a study of 13 patients with Paget disease, MRI detected 7 of 12 underlying cancers, 4 of which were mammographically occult. In this study, MRI also accurately depicted disease extent in 6 patients. Traditional therapy for Paget disease includes mastectomy with or without axillary lymph node dissection. However, the information from a preoperative planning MRI may lead to breast-conserving surgery in many patients (Figures 8, 9).13
Evaluation of One View Findings
Findings seen on one mammographic view with no sonographic correlate can pose a diagnostic challenge; the reported risk of malignancy ranges from 5.7% to 26.3%.14 These findings are most often asymmetries or distortion, and MRI has been particularly useful in this setting historically. However, with the widespread availability of tomosynthesis-guided core needle biopsy, one-view findings can be identified more easily, aiding in preventing unnecessary and costly workups. At our institution, attempts are made to biopsy the area of concern with tomosynthesis, but if the target cannot be confidently identified, MRI may be helpful, as its negative predictive value in this setting is 97.8%.14
Conclusion
The role of breast MRI in the problem-solving arena continues to evolve and expand. It is imperative for radiologists to be familiar with the circumstances under which to recommend MRI to facilitate optimal work up, as it offers much valuable information that can be used to guide patient management. Nevertheless, while future studies may uncover other clinical uses for MRI in breast cancer diagnosis and treatment, it is important to remember that MRI does not currently replace a thorough diagnostic workup that includes mammography and ultrasound.
References
- Preda L, Villa G, Rizzo S et al. Magnetic resonance mammography in the evaluation of recurrence at the prior lumpectomy site after conservative surgery and radiotherapy. Breast Cancer Res. 2006; 8(5) R53.
- Bahl M, Baker JA, Kinsey EN et al. MRI predictors of tumor-positive margins after breast-conserving surgery. Clin Imaging. September-October 2019; 57: 45-49.
- Krammer, J, Price, ER, Jochelson, MS et al. Breast MR imaging for the assessment of residual disease following initial surgery for breast cancer with positive margins. Eur Radiol. 27, 4812–4818 (2017). https://doi.org/10.1007/s00330-017-4823-y.
- Frei KA, Kinkel K, Bonel HM et al. MR imaging of the breast in patients with positive margins after lumpectomy influence of the time interval between lumpectomy and MR imaging. Am J Roentgenol. 2000; 175(6): 1577-1584.
- Fowler AM, Mankoff DA, Joe BN. Imaging neoadjuvant therapy response in breast cancer. Radiology. October 2017; 285(2): 358-375.
- Yeh E, Slanetz P, Kopans DB, et al. Prospective comparison of mammography, sonography, and MRI in patients undergoing neoadjuvant chemotherapy for palpable breast cancer. AJR Am J Roentgenol. March 2005;184(3):868-77. doi: 10.2214/ajr.184.3.01840868. PMID: 15728611.
- Scheel JR, Kim E, Partridge SC, et al. MRI, clinical examination, and mammography for preoperative assessment of residual disease and pathologic complete response after neoadjuvant chemotherapy for breast cancer: ACRIN 6657 Trial. AJR Am J Roentgenol. 2018;210(6):1376-1385. doi:10.2214/AJR.17.18323.
- Orel SG, Weinstein SP, Schnall MD, et al. Breast MR imaging in patients with axillary node metastases and unknown primary malignancy. Radiology. 1999; 212(2) 543-549.
- Olson JA, Morris EA, Van ZEE KJ, et al. Magnetic resonance imaging facilitates breast conservation for occult breast cancer. Ann Surg Oncol. 7(6): 411-415.
- de Bresser J, de Vos, Bent F, et al. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: a systematic review. Eur J Surg Oncol. 2010; 36(2) 114-119.
- Berger N, Luparia A, Di Leo G, et al. Diagnostic performance of MRI versus galactography in women with pathologic nipple discharge: a systematic review and meta-analysis. Am J Roentgenol. 2017; 209: 465-471.
- Bahl M, Baker JA, Greenup RA, et al. Evaluation of pathologic nipple discharge: what is the added diagnostic value of MRI? Ann Surg Oncol. 2015; 22:435-441. https://doi.org/10.1245/s10434-015-4792-9.
- Lim HS, Jeong SJ, Lee JS, et al. RadioGraphics. 2011; 31(7):1973-1987. https://doi.org/10.1148/rg.317115070
- Geiss CS, Chikarmane SA, Sippo DA, et al. Clinical utility of breast MRI in the diagnosis of malignancy after inconclusive or equivocal mammographic diagnostic evaluation. Am J Roentgenol. 2017; 208:6; 1378-1385.
Citation
KB C, A C, M Y.Problem Solving with Breast MRI. Appl Radiol. 2021; (3):17-22.
May 4, 2021