Postoperative Congenital Diaphragmatic Hernia: What the Radiologist Needs to Know
Images





Congenital diaphragmatic hernia (CDH) in which a defect in the diaphragm permits intra-abdominal and/or retroperitoneal structures to move into the thorax, is a complex condition with a prevalence of 1 in 2,500 to 1 in 5,000 live births.1, 2 Knowledge of the short- and long-term sequelae of surgical repair has grown significantly in recent years, and CDH has a high survival rate among children treated at specialized institutions.
Radiology, including MRI, CT, and radiography, plays a vital role in evaluation of the early and late postoperative state; however, postsurgical imaging characteristics have not been thoroughly described in the literature. A deeper understanding of these findings is important for differentiating expected postoperative evolution and sequelae of CDH from complications that may require intervention.
This article briefly reviews preoperative imaging and classification, and then delves into a discussion of postoperative evolution, the imaging appearance of extracorporeal membrane oxygenation (ECMO), expected postoperative findings and unexpected complications involving the vascular, gastrointestinal, pulmonary, neurological, and musculoskeletal systems.
CDH: Prenatal Imaging and Classification
Prenatal imaging, particularly fetal MRI, permits early, accurate diagnosis of CDH by better characterizing the affected anatomy and identifying associated anomalies. Armed with this information, the clinician can better counsel the family and prepare a treatment plan. As increasing experience in prenatal and neonatal care has improved the overall survival rate of CDH, postoperative management is vital to improving patient outcomes and quality of life. In this regard, medical imaging plays an important and essential role in helping to decrease long-term morbidity by identifying postsurgical complications promptly.3
Diaphragmatic hernias are most commonly categorized as either Bochdalek or Morgagni. Bochdalek hernias are typically located posterolaterally in the chest, resulting in contralateral mediastinal shift and pulmonary hypoplasia, predominantly of the ipsilateral lung. This type of hernia occurs more frequently on the left side and may contain the bowel, stomach, spleen, and occasionally the liver. A right-sided Bochdalek hernia may contain the liver, bowel, and occasionally the gallbladder. Rarely, the ipsilateral kidney or adrenal gland may herniate on either side.
Bilateral Bochdalek hernias typically do not have a significant midline shift and usually result in profound hypoplasia of both lungs. While it accounts for only 1% of all cases, bilateral CDH has a dismal prognosis.4, 5 With Bochdalek hernia types, the hernia is occasionally contained by a sac, which is seen as a clear demarcation between the herniated organs/bowel and the apically shifted ipsilateral lung.1 Morgagni hernias are less common, located anteriorly and usually to the right of midline. They often contain both liver and bowel, resulting in a slight leftward mediastinal shift.6
Surgical Approaches to CDH
A multidisciplinary approach to postoperative follow-up has become a mainstay of clinical management. With improving survival, patients with corrected CDH are more prevalent. As such, the multi-organ postsurgical complications of CDH are becoming increasingly recognized. Yet, interpreting postoperative imaging studies in CDH can be challenging. In addition to CDH classification, a thorough understanding of the surgical techniques employed to correct the condition and the expected evolution of CDH over time is required.
The ideal approach to and timing of surgery, which depend largely on the patient’s clinical condition, are a subject of controversy among surgeons. Surgical options include open repair, most commonly via a subcostal incision (Figure 1) or thoracotomy, and thoracoscopy (Figure 2). Historically, CDHs were repaired emergently, leading to some operations being performed on unstable patients.7
From the 1990s onward, the management strategy generally shifted to a delayed operative repair8-10 once the infant was stabilized. The optimal timing for repair depends on many patient factors, including the need for ECMO; some centers are now reporting success with early repair in patients on ECMO.11 During the operation the herniated organs are moved from the thoracic cavity back to the abdomen, followed by diaphragmatic closure via primary repair or placement of a patch. Occasionally, abdominal wall muscle flaps are used for closure. Familiarity with the typical surgical approach at individual institutions will assist radiologists in understanding the postoperative anatomy.
Expected Postoperative Findings and Unexpected Complications
With improved perioperative care, including gentle ventilation, inhaled nitric oxide treatment, and ECMO, a growing number of patients are surviving and requiring long-term management. Many centers advocate close follow-up initially, with gradual spacing of follow-up, often including pre-visit chest radiography, throughout childhood.12 In addition to changes that can be expected after surgery, patients may occasionally experience unexpected complications of the vascular, gastrointestinal, pulmonary, neurological, and musculoskeletal systems related to surgery and/or associated with ECMO. Such complications may present early in the postoperative period to months or years later.
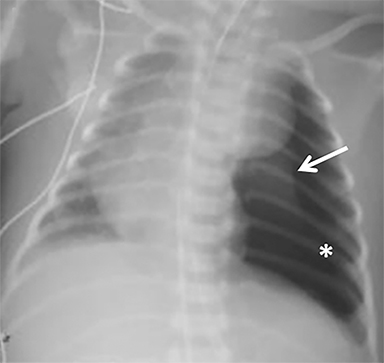
Based on our observations, radiographs typically reveal certain expected postsurgical findings that typically appear chronologically. Immediately after removal of the herniated contents, the ipsilateral chest cavity is air-filled and the hypoplastic lung initially remains collapsed; this normal finding should not be confused with a pneumothorax. While the timeline can vary, the pleural air resorbs and the cavity gradually fills with fluid. With progressive expansion of the ipsilateral lung, the mediastinum begins to shift toward the ipsilateral side and the contralateral lung expands. The pleural fluid eventually resorbs, and mild pleural thickening on the side of the repair may persist long term. This process, depicted in Figure 3, is similar to that which occurs after pulmonary lobectomy.
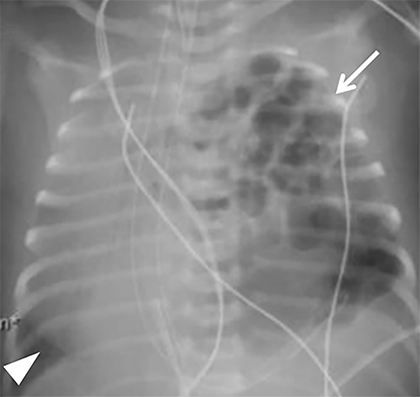
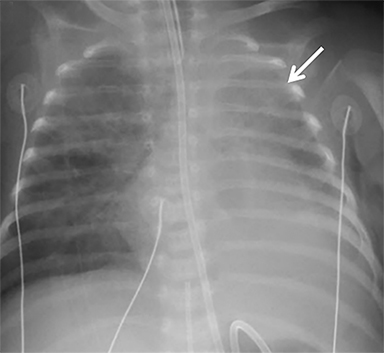
Occasionally, if the pleural fluid continues to accumulate without stabilizing, the mediastinum can shift contralaterally, causing increasing mass effect on the contralateral lung. This urgent finding should be communicated to the surgical team, as it typically requires chest tube placement to relieve the mass effect (Figure 4). In patients with patch repair, the patch can often be seen as a radiopaque linear structure along the diaphragm on radiography or CT, depending on the material used.
Complications Associated with ECMO
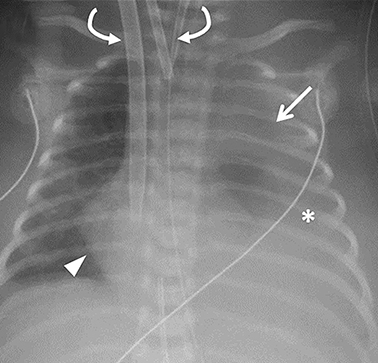
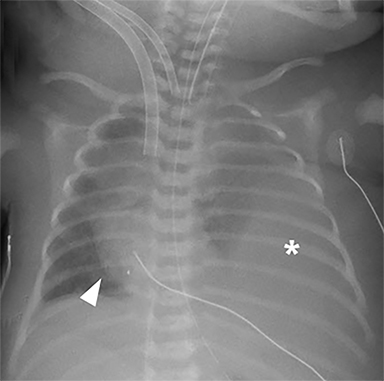
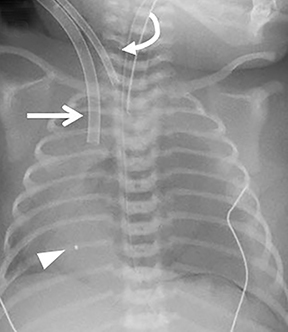
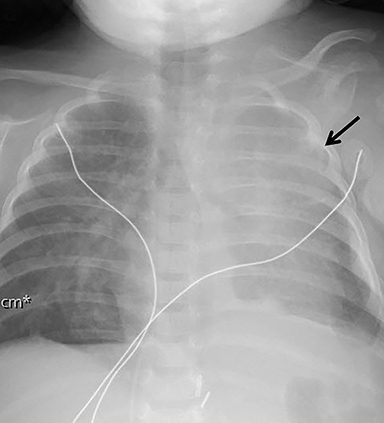
ECMO is typically reserved for patients with severe pulmonary or cardiac compromise refractory to ventilator support and medications. Refractory pulmonary hypertension resulting from pulmonary hypoplasia is the primary cause of mortality in CDH; it is also the primary reason many of these patients require ECMO.13 Depending on the patient’s condition and the institution’s protocol, patients may be placed on ECMO before or after surgery, permitting venous blood bypass to an external membrane oxygenator and then sending it into the arterial or venous circulation. ECMO cannulas can be venoarterial (VA) or venovenous (VV). In VA ECMO, the reperfusion arterial cannula terminates in the right common carotid artery, and the venous cannula is placed in the right internal jugular vein, which terminates in the right atrium (Figure 5). In VV ECMO, a double lumen cannula is typically placed in the right internal jugular vein and terminates in the right atrium (Figure 5B).
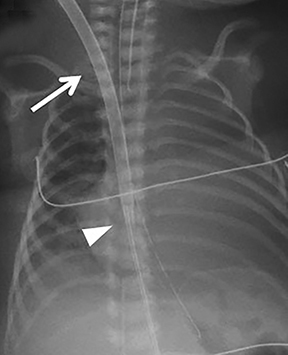
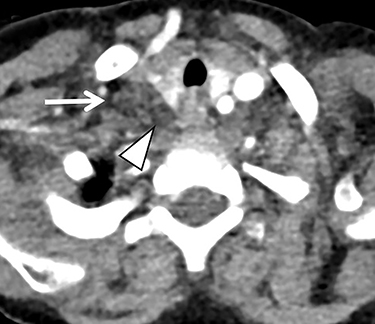
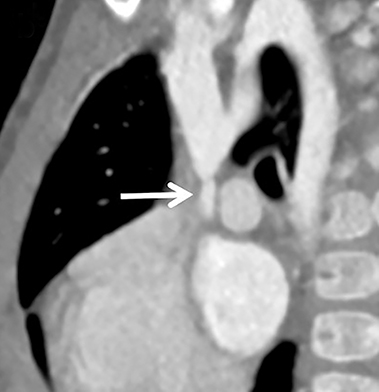
Familiarity with the expected position of the cannulas helps to identify incorrect positioning, which can increase the risk of complications. Cannulas should be actively evaluated for fracture or kinking (Figure 5). Despite the acknowledged survival benefit of ECMO,14 serious neurologic complications may occur. ECMO patients require anticoagulants, putting them at increased risk for intracranial hemorrhage. The large bore of the cannulas can also cause stenosis or occlusion of the internal jugular vein, common carotid artery, and SVC (Figure 6).
Vascular Sequelae
Vascular abnormalities are common in CDH patients, both pre- and postoperatively. The position of vascular support devices on preoperative radiographs vary secondary to the altered anatomy, which should be recognized to avoid unnecessary manipulation. For instance, the umbilical venous catheter may deviate significantly to the left of the spine, often in the left hemithorax in cases of liver-up left CDH.15 The umbilical arterial catheter may be positioned to the right of the spine, or even to the right of the umbilical venous catheter. These findings generally do not indicate a situs abnormality, but rather are related to deviation and rotation of the vasculature secondary to the hernia.
In patients with liver-up CDH, hepatic vasculature positioned above the diaphragm can result in altered hepatic perfusion from vascular congestion, which may persist in the postoperative period and is usually inconsequential (Figure 7). Infrequently. serious complications may develop; eg, IVC or hepatic veins may become kinked in a right CDH. After surgery, the kinked IVC may be further compressed by the liver,16 leading to chronic narrowing and, potentially, thrombosis. Patients are also predisposed to form thrombus in the aorta or other major arteries owing to the arterial catheters, such as UAC and ECMO cannulas.
Complications Directly Related to CDH Repair
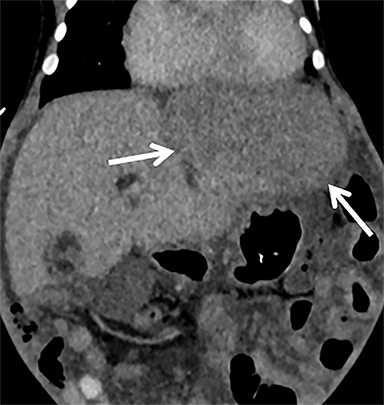
Complications of CDH repair may occur intraoperatively or postoperatively during or after the hospital stay. Intraoperative hemorrhage or organ injury while reducing herniated structures during CDH repair can rarely lead to abdominal compartment syndrome and may require delayed abdominal closure.16 Patients may develop primary infection, including a patch abscess, which is best appreciated on CT (Figure 8).
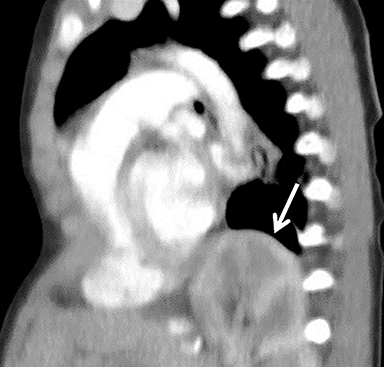
Hernia recurrence typically presents later in childhood but can rarely present during the initial hospital stay (Figure 9). The bowel is the most common organ to herniate, though usually to a lesser extent than the original hernia. Many recurrences are asymptomatic; surveillance chest radiography can be performed to identify them early. Patients may present with mild chest pain or, more seriously, respiratory failure or bowel obstruction.12
Gastrointestinal (GI) Sequelae
Functional sequelae associated with the GI system are expected in most postoperative cases. Gastroesophageal reflux (GER) has been reported in up to 84% of patients,17 usually presenting early on and often persisting well into childhood and even adulthood. Many theories have been proposed to explain the increased risk of GER. These include esophageal dysmotility and dilatation from prior mass effect, shortening of the esophagus, increased postsurgical intra-abdominal pressure, altered diaphragmatic motion from a weakened crus, disruption of the angle of His, or any combination thereof.18-21 Patients with unrecognized GER can develop feeding aversions and may require a gastrostomy tube to prevent failure to thrive. Therefore, early diagnosis is essential to providing an improved quality of life.
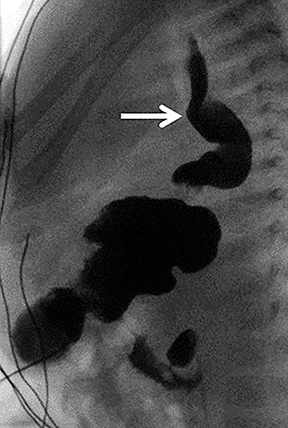
Although GER is a clinical diagnosis, upper GI fluoroscopy can further delineate the process. In addition to the presence and extent of reflux across the GE junction, a dilated tortuous esophagus with abnormal peristalsis may be visualized (Figure 10). Routine radiographic assessment for an air-filled, dilated esophagus, usually best seen on a lateral view, should be performed. A fundoplication is often performed to reduce the risk of reflux and aspiration that would otherwise further complicate already reduced pulmonary function. Fundoplication also reduces the risk of esophagitis and Barrett esophagus, which studies have shown to be a potential complication in adult survivors of CDH repair.22, 23
Bowel obstruction may develop secondary to adhesions, volvulus, or recurrent hernia.24 While radiography may suffice in some instances, cross-sectional imaging is often needed for thorough evaluation. Abdominal radiographs should be assessed for air-fluid levels, bowel distention, pneumatosis, and pneumoperitoneum, as 10-20% of patients can develop bowel obstruction.25 Some suggest that CDH survivors have an increased likelihood of forming adhesions due to increased intra-abdominal pressure and slowed peristalsis from surgery.22 Patients with recurrent hernia can develop a closed loop obstruction and even perforation. Recognizing these on imaging is critical; recurrent hernia is one of the most common indications for a second surgical intervention.25 Similarly, in utero obstruction of herniated bowel with perforation can result in meconium spillage, causing pleuritis and peritonitis.26, 27 The meconium calcifies and appears as coarse pleural and/or peritoneal densities on radiographs.
The vast majority of CDH patients have some degree of malrotation of the bowel, which can rarely predispose to midgut volvulus.28, 29 Some surgeons may perform a preventative Ladd procedure at the time of hernia repair, although the frequency of this procedure varies with severity of the malrotation and surgeon preference. These findings may be appreciated on radiographs as an altered position of the post-pyloric feeding tube. An association between CDH and pyloric stenosis has also been described30 and can be seen in 1.2% of CDH patients.31
Pulmonary Sequelae
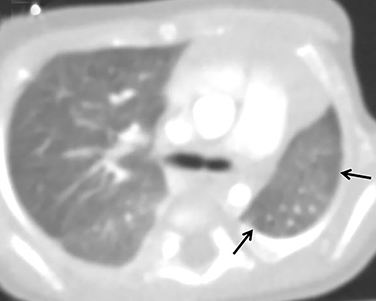
Pulmonary sequelae of CDH are seen in the majority of CDH survivors. Hypoplasia of the ipsilateral lung and, to a lesser degree, the contralateral lung, are expected and important prognostic factors (Figure 11). In utero mass effect on fetal lungs also leads to structural abnormalities of the bronchi, alveoli, and vascular bed.32 Mosaic attenuation related to air trapping and/or perfusion abnormalities may be seen on CT (Figure 11). Furthermore, superimposed ventilator-induced lung injury can lead to the appearance of fibrotic lung changes. Chronic pleural thickening on the side of the hernia repair is often seen and should not be mistaken for a pleural effusion.
Hypoperfusion of the affected lung can be seen on a ventilation-perfusion scan secondary to hypoplastic pulmonary vasculature.32 Patients may eventually develop pulmonary hypertension, suggested on chest imaging by pulmonary artery enlargement, right ventricular hypertrophy, or signs of right heart failure.33 Significant hypoxic respiratory failure from pulmonary hypoplasia and pulmonary hypertension is a leading cause of short- and long-term morbidity.34 Pulmonary function tests may demonstrate obstructive and/or restrictive airway disease.32
Neurological and Musculoskeletal Sequelae
Imaging can also reveal neurological abnormalities associated with CDH. Radhakrishnan, et al, suggested an association of brain injury with severity of pulmonary hypoplasia and hypoxia, and with right-sided hernias.35 The most frequently encountered finding on imaging was enlarged extra-axial spaces in the postnatal brain; however, ventriculomegaly and white matter injury were also identified.35 Others have described bifrontal atrophy and ventricular dilatation on imaging during childhood.36 Furthermore, ECMO itself can cause enlarged extra-axial spaces in addition to complications such as infarction, seizures, and intracranial hemorrhage associated with anticoagulation.37
Musculoskeletal findings in postoperative patients include scoliosis and chest wall deformities, including pectus excavatum and chest wall hypoplasia.24 Mild to moderate scoliosis is frequently seen; progression can compromise chest wall compliance and quality of life, and often requires surgical intervention.38 Pectus excavatum is the most common chest wall deformity, narrowing the thoracic cavity and preventing complete lung expansion due to decreased chest wall compliance in severe cases.39
Conclusion
CDH is a complex condition that can be addressed through various surgical strategies. Patient management of CDH survivors is a lifelong process. A thorough understanding of the imaging appearances of pre- and postoperative anatomy, as well as the expected postoperative evolution of CDH, can help the pediatric radiologist to quickly distinguish expected findings from unexpected complications.
References
- Kline-Fath BM. Current advances in prenatal imaging of congenital diaphragmatic [corrected] hernia. Pediatric radiology. Jan 2012;42 Suppl 1:S74-90. doi:10.1007/s00247-011-2183-3
- Losty PD. Congenital diaphragmatic hernia: where and what is the evidence? Semin Pediatr Surg. Oct 2014;23(5):278-82. doi:10.1053/j.sempedsurg.2014.09.008
- Takayasu H, Masumoto K, Jimbo T, et al. Analysis of risk factors of long-term complications in congenital diaphragmatic hernia: A single institution’s experience. Asian J Surg. Jan 2017;40(1):1-5. doi:10.1016/j.asjsur.2015.02.005
- Botden SM, Heiwegen K, van Rooij IA, et al. Bilateral congenital diaphragmatic hernia: prognostic evaluation of a large international cohort. J Pediatr Surg. Sep 2017;52(9):1475-1479. doi:10.1016/j.jpedsurg.2016.10.053
- Kays DW, Islam S, Larson SD, Perkins J, Talbert JL. Long-term maturation of congenital diaphragmatic hernia treatment results: toward development of a severity-specific treatment algorithm. Ann Surg. Oct 2013;258(4):638-44; discussion 644-5. doi:10.1097/SLA.0b013e3182a53c49
- Chavhan GB, Babyn PS, Cohen RA, Langer JC. Multimodality imaging of the pediatric diaphragm: anatomy and pathologic conditions. Radiographics. Nov 2010;30(7):1797-817. doi:10.1148/rg.307105046
- Harting MT, Lally KP. Surgical management of neonates with congenital diaphragmatic hernia. Semin Pediatr Surg. May 2007;16(2):109-14. doi:10.1053/j.sempedsurg.2007.01.007
- Wung JT. Respiratory management for low-birth-weight infants. Crit Care Med. Sep 1993;21(9 Suppl):S364-5.
- Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med. Oct 1 2002;166(7):911-5. doi:10.1164/rccm.200204-304CC
- Sakai H, Tamura M, Hosokawa Y, Bryan AC, Barker GA, Bohn DJ. Effect of surgical repair on respiratory mechanics in congenital diaphragmatic hernia. J Pediatr. Sep 1987;111(3):432-8.
- Dassinger MS, Copeland DR, Gossett J, et al. Early repair of congenital diaphragmatic hernia on extracorporeal membrane oxygenation. J Pediatr Surg. Apr 2010;45(4):693-7. doi:10.1016/j.jpedsurg.2009.08.011
- Jancelewicz T, Chiang M, Oliveira C, Chiu PP. Late surgical outcomes among congenital diaphragmatic hernia (CDH) patients: why long-term follow-up with surgeons is recommended. J Pediatr Surg. May 2013;48(5):935-41. doi:10.1016/j.jpedsurg.2013.02.005
- Tiruvoipati R, Vinogradova Y, Faulkner G, Sosnowski AW, Firmin RK, Peek GJ. Predictors of outcome in patients with congenital diaphragmatic hernia requiring extracorporeal membrane oxygenation. J Pediatr Surg. Aug 2007;42(8):1345-50. doi:10.1016/j.jpedsurg.2007.03.031
- Kays DW, Islam S, Richards DS, Larson SD, Perkins JM, Talbert JL. Extracorporeal life support in patients with congenital diaphragmatic hernia: how long should we treat? J Am Coll Surg. Apr 2014;218(4):808-17. doi:10.1016/j.jamcollsurg.2013.12.047
- Chang PT, Taylor GA. Umbilical venous catheter malposition and errors in interpretation in newborns with Bochdalek hernia. Pediatric radiology. Jul 2015;45(7):982-8. doi:10.1007/s00247-014-3275-7
- Barroso C, Correia-Pinto J. Perioperative Complications of Congenital Diaphragmatic Hernia Repair. Eur J Pediatr Surg. Apr 2018;28(2):141-147. doi:10.1055/s-0038-1632374
- Arena F, Romeo C, Baldari S, et al. Gastrointestinal sequelae in survivors of congenital diaphragmatic hernia. Pediatr Int. Feb 2008;50(1):76-80. doi:10.1111/j.1442-200X.2007.02527.x
- Zabrocki LA, Brogan TV, Statler KD, Poss WB, Rollins MD, Bratton SL. Extracorporeal membrane oxygenation for pediatric respiratory failure: Survival and predictors of mortality. Crit Care Med. Feb 2011;39(2):364-70. doi:10.1097/CCM.0b013e3181fb7b35
- Marseglia L, Manti S, D’Angelo G, et al. Gastroesophageal reflux and congenital gastrointestinal malformations. World J Gastroenterol. Jul 28 2015;21(28):8508-15. doi:10.3748/wjg.v21.i28.8508
- St Peter SD, Valusek PA, Tsao K, Holcomb GW, 3rd, Ostlie DJ, Snyder CL. Abdominal complications related to type of repair for congenital diaphragmatic hernia. J Surg Res. Jun 15 2007;140(2):234-6. doi:10.1016/j.jss.2007.03.018
- Su W, Berry M, Puligandla PS, Aspirot A, Flageole H, Laberge JM. Predictors of gastroesophageal reflux in neonates with congenital diaphragmatic hernia. J Pediatr Surg. Oct 2007;42(10):1639-43. doi:10.1016/j.jpedsurg.2007.05.016
- Vanamo K, Rintala RJ, Lindahl H, Louhimo I. Long-term gastrointestinal morbidity in patients with congenital diaphragmatic defects. J Pediatr Surg. Apr 1996;31(4):551-4.
- Steven MJ, Fyfe AH, Raine PA, Watt I. Esophageal adenocarcinoma: a long-term complication of congenital diaphragmatic hernia? J Pediatr Surg. Jul 2007;42(7):E1-3. doi:10.1016/j.jpedsurg.2007.04.026
- Jancelewicz T, Vu LT, Keller RL, et al. Long-term surgical outcomes in congenital diaphragmatic hernia: observations from a single institution. J Pediatr Surg. Jan 2010;45(1):155-60; discussion 160. doi:10.1016/j.jpedsurg.2009.10.028
- Nobuhara KK, Lund DP, Mitchell J, Kharasch V, Wilson JM. Long-term outlook for survivors of congenital diaphragmatic hernia. Clin Perinatol. Dec 1996;23(4):873-87.
- Christopher TD, Effmann EL, Filston HC. Meconium peritonitis and pleuritis: a clue to perforation of an incarcerated Bochdalek hernia in a neonate. J Pediatr Surg. May 1990;25(5):558-9.
- Kimball D, Smith W. Meconium peritonitis with thoracic extension. AJR Am J Roentgenol. Jan 1985;144(1):113-4. doi:10.2214/ajr.144.1.113
- Rescorla FJ, Shedd FJ, Grosfeld JL, Vane DW, West KW. Anomalies of intestinal rotation in childhood: analysis of 447 cases. Surgery. Oct 1990;108(4):710-5; discussion 715-6.
- Stolar CJH, Dillon PW. Chapter 60 - Congenital Diaphragmatic Hernia and Eventration A2 - Grosfeld, Jay L. In: O’Neill JA, Coran AG, Fonkalsrud EW, Caldamone AA, eds. Pediatric Surgery (Sixth Edition). Mosby; 2006:931-954.
- Al-Salem AH, Grant C, Khwaja S. Infantile hypertrophic pyloric stenosis and congenital diaphragmatic hernia. J Pediatr Surg. Jun 1990;25(6):607-8.
- Abdullah F, Zhang Y, Sciortino C, et al. Congenital diaphragmatic hernia: outcome review of 2,173 surgical repairs in US infants. Pediatr Surg Int. Dec 2009;25(12):1059-64. doi:10.1007/s00383-009-2473-0
- Peetsold MG, Heij HA, Kneepkens CM, Nagelkerke AF, Huisman J, Gemke RJ. The long-term follow-up of patients with a congenital diaphragmatic hernia: a broad spectrum of morbidity. Pediatr Surg Int. Jan 2009;25(1):1-17. doi:10.1007/s00383-008-2257-y
- Schwartz IP, Bernbaum JC, Rychik J, Grunstein M, D’Agostino J, Polin RA. Pulmonary hypertension in children following extracorporeal membrane oxygenation therapy and repair of congenital diaphragmatic hernia. J Perinatol. Apr-May 1999; 19(3):220-6.
- Wynn J, Krishnan U, Aspelund G, et al. Outcomes of congenital diaphragmatic hernia in the modern era of management. J Pediatr. Jul 2013;163(1):114-9 e1. doi:10.1016/j.jpeds.2012. 12.036
- Radhakrishnan R, Merhar SL, Su W, et al. Prenatal Factors Associated with Postnatal Brain Injury in Infants with Congenital Diaphragmatic Hernia. AJNR Am J Neuroradiol. Dec 21 2017;doi:10.3174/ajnr.A5500
- Lund DP, Mitchell J, Kharasch V, Quigley S, Kuehn M, Wilson JM. Congenital diaphragmatic hernia: the hidden morbidity. J Pediatr Surg. Feb 1994;29(2):258-62; discussion 262-4.
- van Heijst AF, de Mol AC, Ijsselstijn H. ECMO in neonates: neuroimaging findings and outcome. Semin Perinatol. Mar 2014;38(2):104-13. doi: 10.1053/j.semperi.2013.11.008
- Antiel RM, Riley JS, Cahill PJ, et al. Management and outcomes of scoliosis in children with congenital diaphragmatic hernia. J Pediatr Surg. Dec 2016;51(12):1921-1925. doi:10.1016/j.jpedsurg.2016.09.013
- Koumbourlis AC, Stolar CJ. Lung growth and function in children and adolescents with idiopathic pectus excavatum. Pediatr Pulmonol. Oct 2004;38(4):339-43. doi:10.1002/ppul.20062
Citation
AP G, D F, D K, J Q, JN K.Postoperative Congenital Diaphragmatic Hernia: What the Radiologist Needs to Know. Appl Radiol. 2020; (6):34-41.
November 6, 2020