Post-traumatic Retrograde Urethrography: A Review of Acute Findings and Chronic Complications
Images






While injuries to the urethra are relatively rare compared to other segments of the genitourinary tract, urethral injury can be seen in 4-24% of male patients with pelvic fractures.1-4 Although rarely life-threatening, urethral injuries can lead to significant long-term morbidity.1,2 Retrograde urethrography (RUG) continues to be the best initial diagnostic study for evaluating acute male urethral trauma and post-traumatic complications.5,6 Accurate technique in performing these procedures in both the pre- and postoperative settings can be challenging, and knowledge of normal and abnormal findings is essential to guiding management and treatment. This review article will highlight normal urethral anatomy, describe the appropriate techniques for conducting RUG examinations, accompanied by practical troubleshooting strategies; and review post-traumatic pathologies.
Normal Anatomy
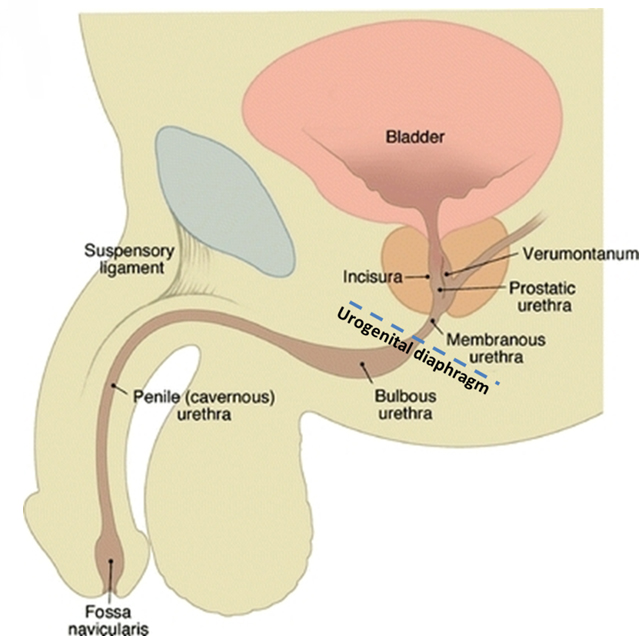
The male urethra extends from the external meatus to the urinary bladder with a length ranging from 17 to 24 cm (reported mean 22 ± 2.4 cm).7 The urethra is divided into anterior and posterior segments separated by the urogenital diaphragm (Figure 1). The anterior urethra extends from the external meatus to the urogenital diaphragm and is subdivided into a proximal bulbous segment and a distal penile segment, spanning approximately 16 cm. The distal-most aspect of the anterior urethra is termed the fossa navicularis; it is approximately 1 to 1.5 cm in length. The Cowper’s glands, located within the urogenital diaphragm, drain into the proximal bulbous segment. The periurethral Littré glands are located in the dorsal aspect of the penile urethra and distal bulbous urethra.
The posterior urethra extends from the urogenital diaphragm to the urinary bladder, measuring approximately 5 cm. It is subdivided into a proximal prostatic segment and a distal membranous segment. The verumontanum is a 1 cm-long ovoid mound located in the posterior wall of the prostatic urethra, the center of which contains the prostatic utricle. The prostatic utricle is flanked bilaterally by the ejaculatory ducts. The internal urethral sphincter spans the length of the bladder neck to just proximal to the verumontanum. The intrinsic urethral sphincter is located distal to the verumontanum within the distal prostatic urethra. The extrinsic sphincter arises from the levator ani complex and surrounds the membranous urethra.
Indications
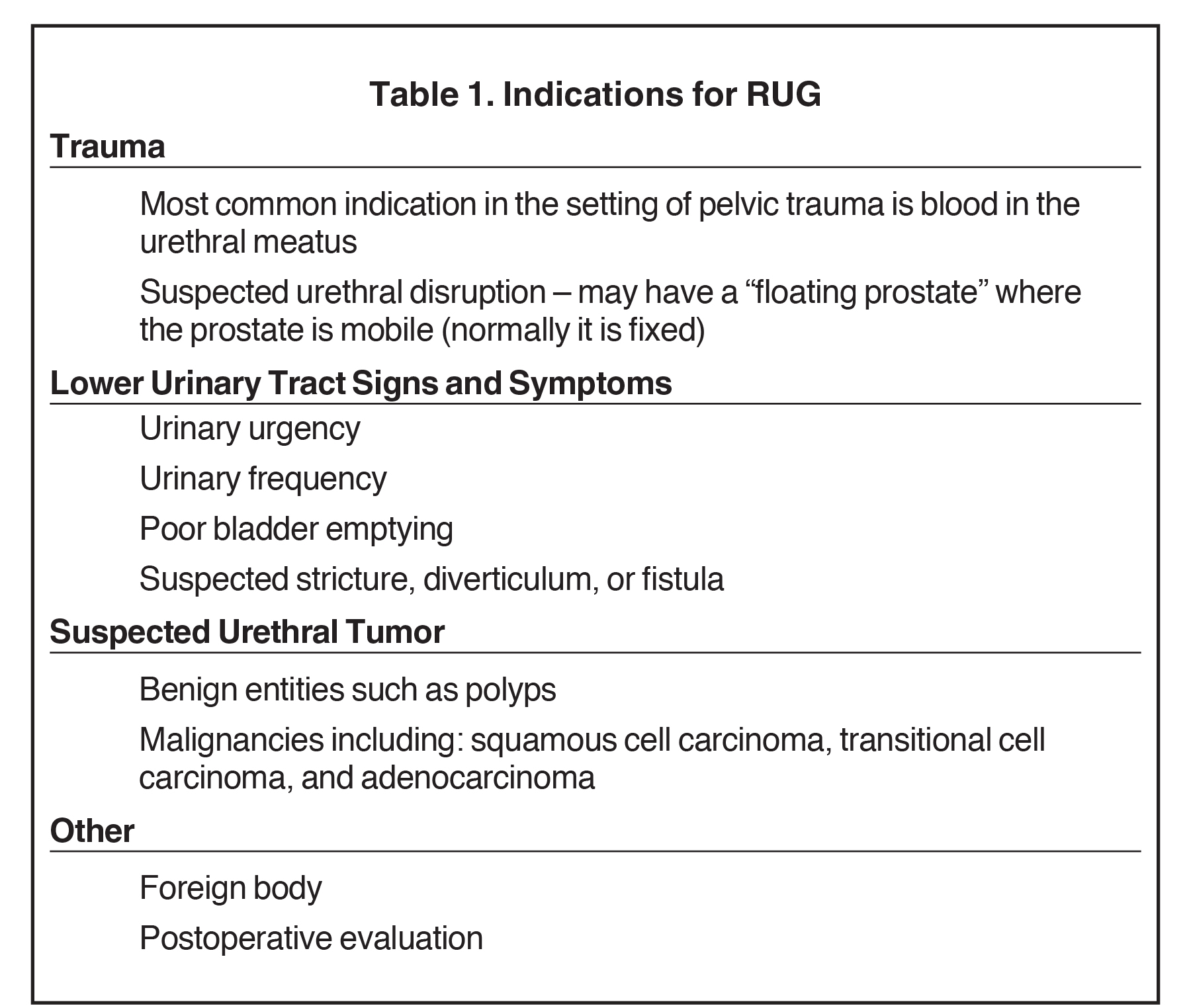
Indications for RUG include trauma, lower urinary tract structural abnormalities, urethral masses, and postoperative evaluation (Table 1). The most common indication is trauma, which on physical examination traditionally presents with blood within the urethral meatus or a mobile prostate gland in the case of urethral disruption.8,9 Lower urinary tract structural pathologies that warrant RUG include abnormalities such as urethral strictures, urethral diverticulum, or urethral fistula which may present with urinary urgency, weak urinary stream, and poor bladder emptying.10
Contraindications
While no absolute contraindications for RUG exist, there are several relative contraindications (Table 1). Allergy to iodinated contrast is one, and while a properly performed RUG should not result in contrast entry into the vascular system, it may result in venous intravasation.11 In cases of reported allergy to iodinated contrast, institutional protocols should be followed, which often include premedication with corticosteroids and antihistamines is recommended.11 Active urinary tract infection is also considered a relative contraindication, and in a non-emergent setting, urine laboratory analysis and urine culture should be performed prior to RUG.11 Unstable trauma patients may require life-saving procedures to address more emergent injuries prior to urethral evaluation. Lastly, recent instrumentation is a relative contraindication and a thorough clinical and surgical history should be obtained prior to the examination.
Technique
At our institution, we use a 14 French Foley catheter for most RUGs. Smaller catheters, ranging from 8 to 12 French, may be used with smaller urethras. With distal-most penile urethral strictures, such as those at the meatus, we employ pediatric 5 French feeding tubes, although these lack inflatable balloons for adequate anchoring and must be held in place by the radiologist during the examination. Prior to beginning a RUG, the physician should perform a preoperative equipment check. Inflate the Foley catheter balloon with 2 cc of air using a 5 cc Luer lock syringe, and then flush the catheter with contrast to ensure patency and remove air bubbles. The use of lubricating jelly with the Foley catheter is controversial and is not recommended by some authors.12,13 We do not use lubrication, as it is likely to dislodge the catheter. Moreover, the contrast itself used to flush the Foley catheter acts as a lubricant, to reduce patient discomfort.
Next, place the patient in a supine, 45-degree oblique position with the penis situated in a plane oriented laterally over the proximal thigh. Sterilize the urethral meatus with an antiseptic iodine-based solution. Then insert the catheter gently into the fossa navicularis and inflate the balloon with 2 cc of air. Inform the patient prior to balloon inflation that some discomfort may result.
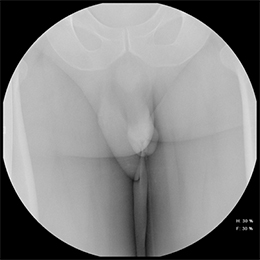
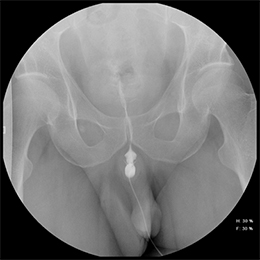
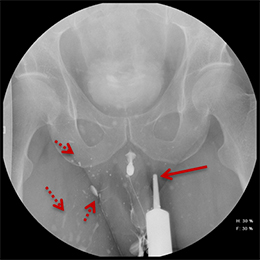
A pre-injection scout radiograph should be obtained (Figure 2). Using gentle pressure, we hand inject 18% iodinated, water-soluble contrast through the catheter in a retrograde fashion with the penis in slight traction while concurrently obtaining static radiographs. The volume and pressure needed for contrast injection depends on the degree of obstruction; the examiner should determine volume and pressure while evaluating spot radiographs throughout the study. The physician should instill enough contrast to visualize a full column of contrast spanning the urethra from the fossa navicularis to the urinary bladder (Figure 1). If overlapping structures obscure pathology, position the patient in a more oblique fashion. Following imaging, deflate the balloon and remove the catheter. Instruct the patient to stand in an oblique position and void into a urinal container, and obtain additional images to assess for pathology at the distal-most urethra and fossa navicularis. Finally, obtain a post-voiding image of the bladder to document the presence of residual contrast.
Troubleshooting Strategies
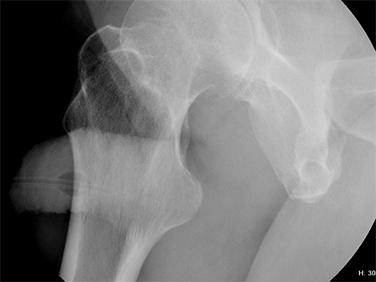
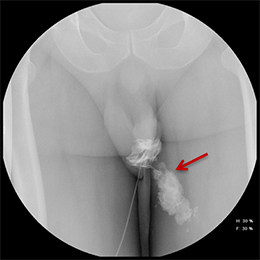
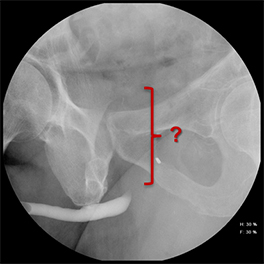
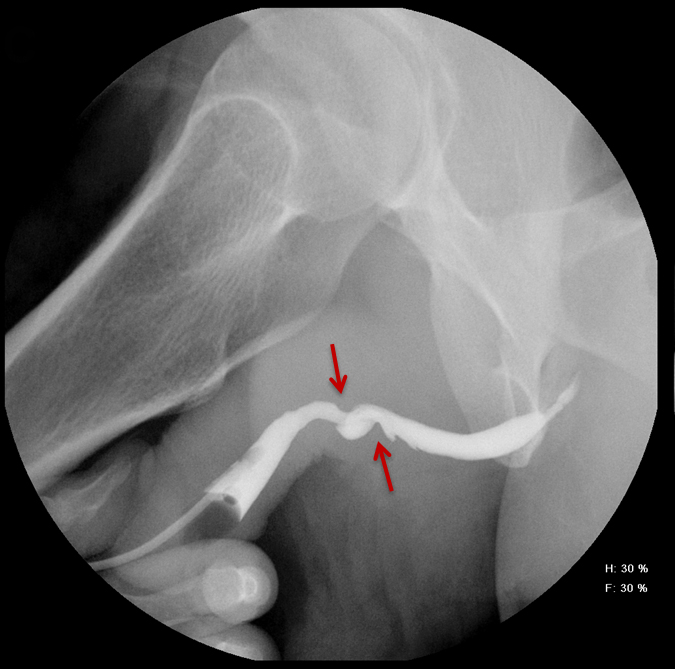
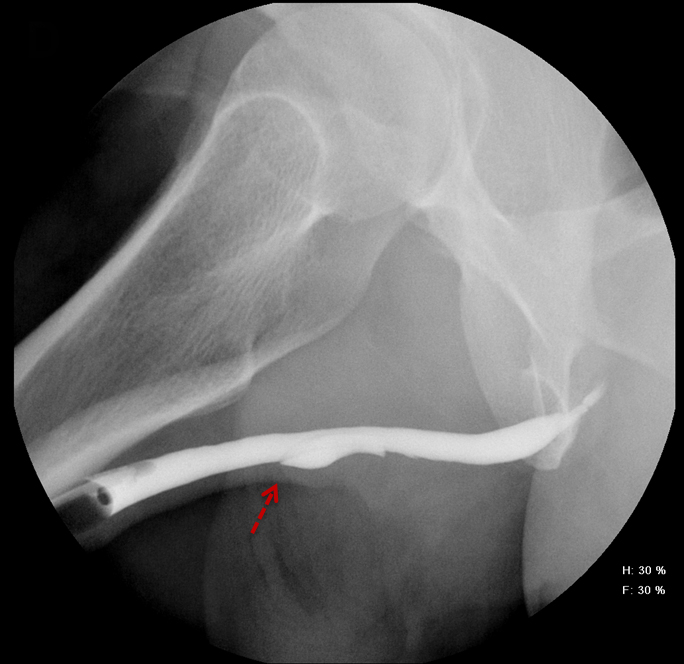
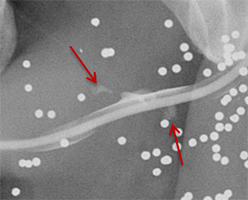
Balloon slippage may occur when pressure from hand injection displaces the balloon and expels the catheter from the penis. This may be secondary to an inadequately sized catheter. Should this occur, insert a larger Foley catheter to reduce future slippage (Figure 3). In the setting of a narrow stricture, hand injection may cause increased intracatheter pressures, disconnecting the syringe from the catheter. If this occurs, ensure proper sealing of the tubing before hand-injecting with the catheter held firmly in place (Figure 3).
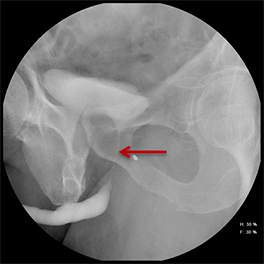
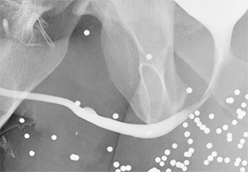
Increased extrinsic sphincter contraction secondary to spasm may result in non-opacification of the posterior urethra. In this event, instruct the patient to deeply inhale and exhale, and hand inject contrast with gentle pressure. In the absence of pathology, contrast should freely cross the posterior urethra into the urinary bladder (Figure 4). Improper penile positioning may result in a foreshortened and distorted urethral anatomy appearance that may mimic strictures and other urethral pathology. By applying moderate traction to the penis and obtaining images in the urethral long axis, distortion should resolve (Figure 4).
Complications
Allergy to iodinated contrast is a relative contraindication. In a properly performed RUG, contrast should enter the vascular system; however, an anaphylactoid reaction may result from the rare case of intravascular contrast intravasation, identified as opacification of the corpora and draining veins. Closely monitor the patient for an anaphylactoid reaction when such intravasation is identified. As a prophylactic measure, follow institutional protocols which often include premedication of patients with allergies to iodinated contrast, and may include the use of antihistamines and corticosteroids prior to RUG.
Urinary tract infections are also rare following RUGs performed with proper sterile technique. Patients may experience dysuria, a burning sensation while urinating, or fever. Workup includes urinalysis and urine culture, with symptomatic patients undergoing empiric antibiotic therapy.
Iatrogenic urethral injury is an extremely rare complication of RUG in patients with a structurally normal urethra. With distal urethral tears, attempting to insert the Foley catheter beyond the tear may cause tear propagation or urethral perforation; the examination should be promptly discontinued.
Normal Studies
A normal RUG study should demonstrate a continuous contrast column extending from the fossa navicularis to the urinary bladder (Figure 1). The penile urethra should be mostly uniform in caliber with the bulbous urethra slightly larger than the penile urethra. At the bulbomembranous junction there should be a smooth change in caliber of the smaller membranous urethra into the larger bulbous urethra. With inadequate distention of the urethra, the verumontanum is visualized as an ovoid filling defect in the posterior prostatic urethra approximately 1.5 cm proximal to the bulbomembranous junction. Following voiding, the urethra and urinary bladder should be essentially free of contrast.
Urethral Trauma
Blunt Urethral Trauma
Blunt urethral trauma accounts for most traumatic urethral injuries, and generally occurs in the anterior urethra, owing to its unprotected nature and increased mobility.8,14 Anterior urethral injuries are also associated with pelvic fracture and are the result of straddle perineal injuries secondary to bulbous urethra impaction onto the pubis symphysis.14,15 In penile fractures, anterior urethral injury has an incidence of up to 20%.14
Unlike anterior urethral injuries, most posterior urethral trauma occurs in the setting of pelvic fractures, with an incidence of 4 – 24%.15,16 With pelvic fractures, the blunt urethral trauma is typically secondary to direct injury.17 In the absence of pelvic fracture, posterior urethral injuries usually result from shearing forces, causing urethral stretching between points of relative fixation, including the puboprostatic ligament or the fascia of the urogenital diaphragm.14,17
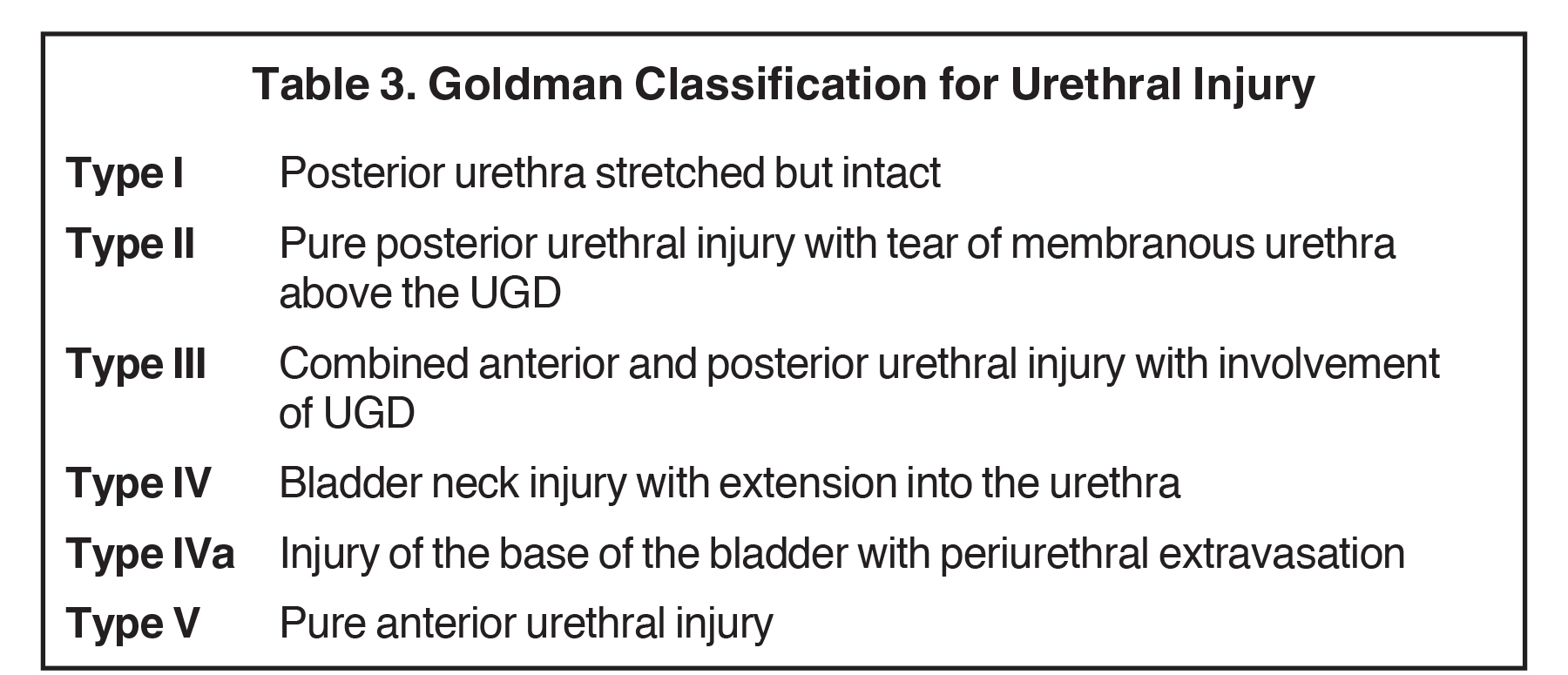
Urethral contusions represent a low-grade injury and are typically occult on RUG, with patients traditionally treated conservatively, including pain management.12 Higher-grade blunt urethral injuries have a variety of imaging characteristics and can be categorized using the Goldman Classification, which divides urethral injury into subtypes based on the anatomic segment(s) involved (Table 2). Accurate classification is crucial to guiding optimal treatment planning.
Type I injuries are relatively uncommon, accounting for 10-15% of blunt urethral trauma.13 Injury is isolated to the posterior urethra, which is stretched but remains intact without evidence of extraluminal contrast extravasation. Treatment is conservative, with suprapubic or urethral catheterization.12,13,18 Type II injuries also account for 10-15% of blunt urethral trauma.13 Tearing is present, and isolated to the posterior urethra, resulting in contrast extravasation. Treatment usually consists of suprapubic or urethral catheterization for partial (< 25% thickness) tears, and realignment or urethroplasty for high-grade (> 75% thickness) tears.5,12,13,18
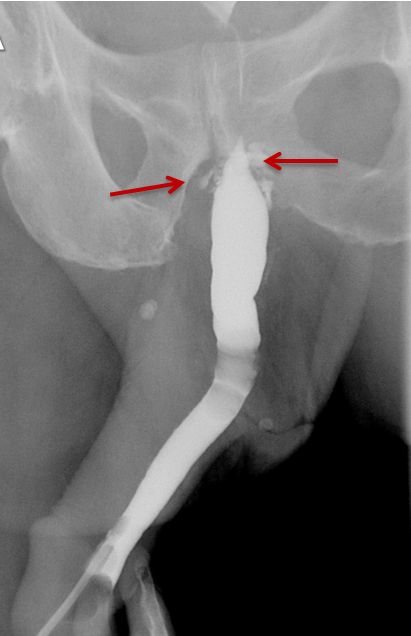
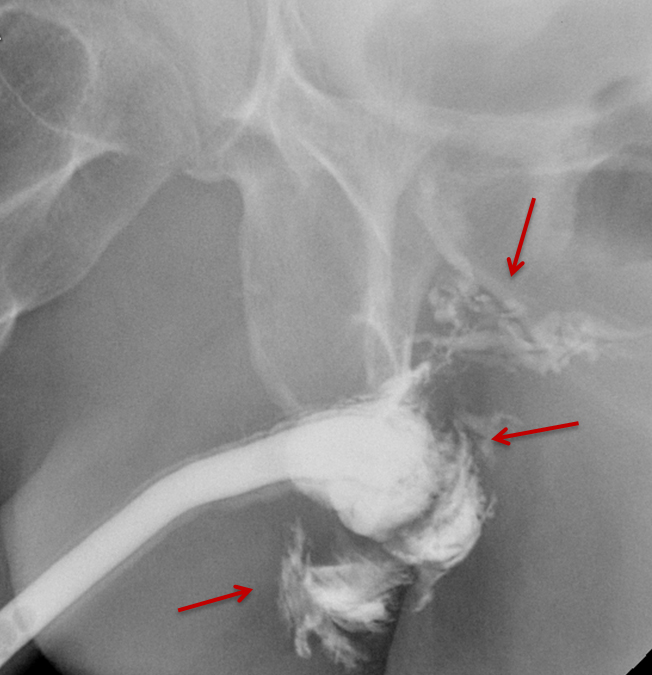
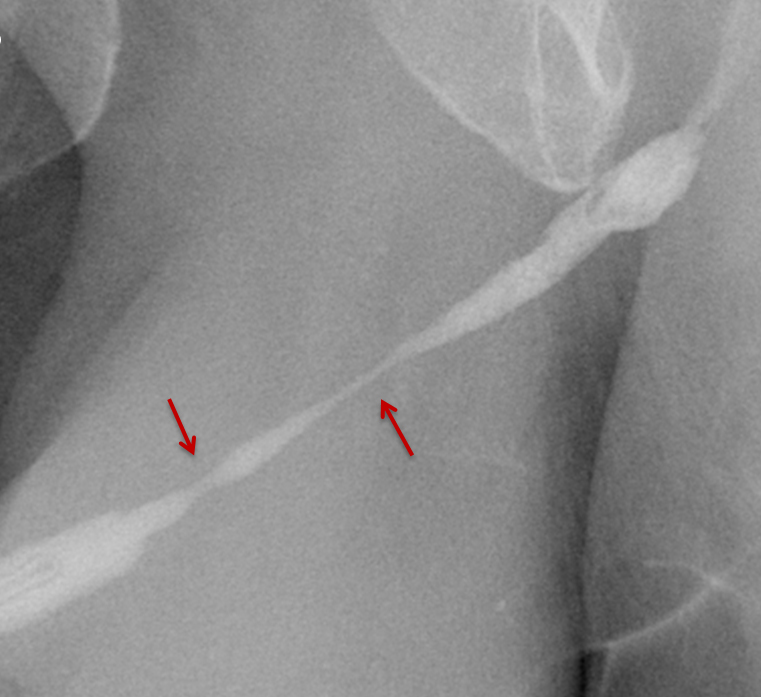
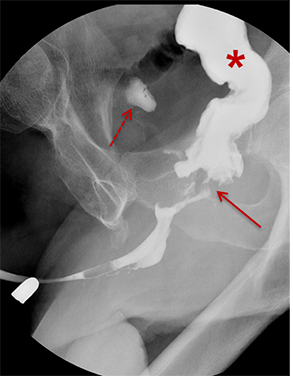
Type III injuries are most common, accounting for 66 – 85% of all blunt urethral trauma.5 They are defined as combined anterior and posterior urethral injury with involvement of the urogenital diaphragm, resulting in contrast extravasation from these areas (Figure 5). Treatment for type III injuries is similar to type II injuries.5,12,13,18 Type IV injuries are relatively rare and are defined as bladder neck injury with extension into the urethra. 12,13,18 Patients with type IV injuries may present with incontinence secondary to injury to the detrusor musculature. Type IVa injuries should not be confused with type IV injuries; Type IVa injuries are defined as a pure extraperitoneal injury to the bladder base and have lesser association with incontinence than type IV injuries. 12,13,18
Type V injuries are isolated to the anterior urethra and typically involve the bulbous urethra as a result of straddle injury (Figure 5). 12,13,18,19 If the Buck fascia is intact, extravasation is limited to the space between the Buck fascia and tunica albuginea. If the Buck fascia is disrupted, extravasation will be present and located between the confines of the Colle fascia. Treatment is similar to type II and III injuries. 13,18,19
Penetrating Urethral Injury
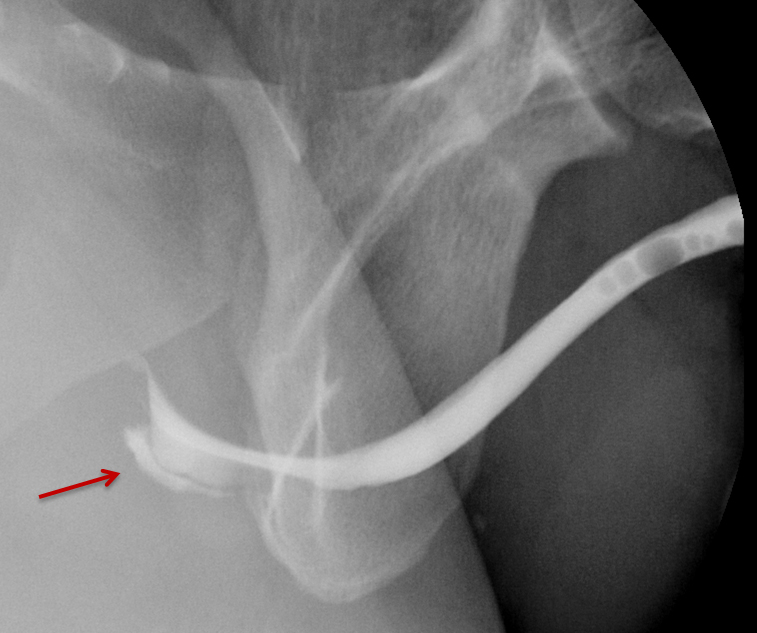
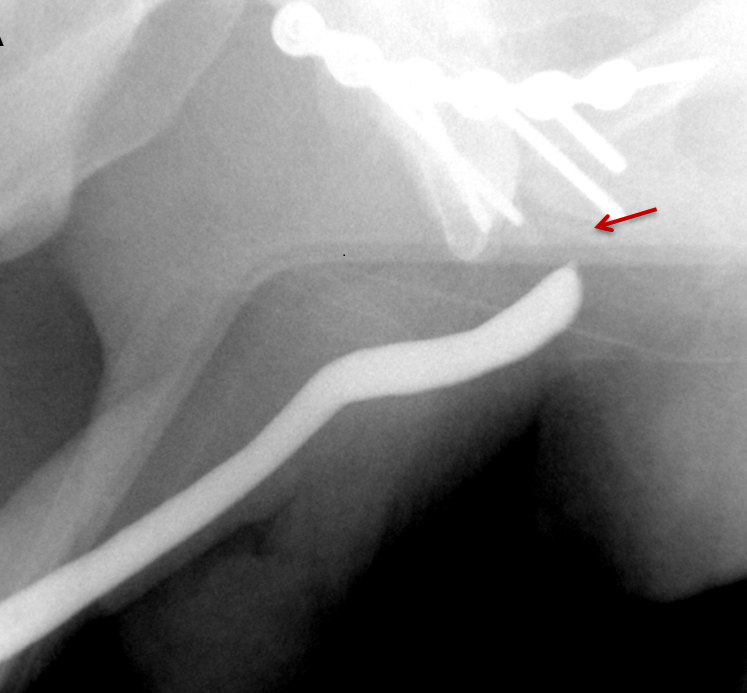
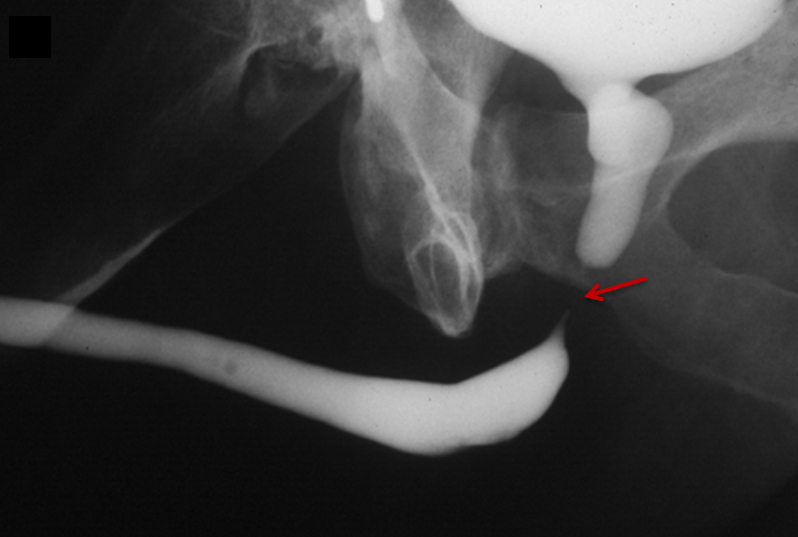
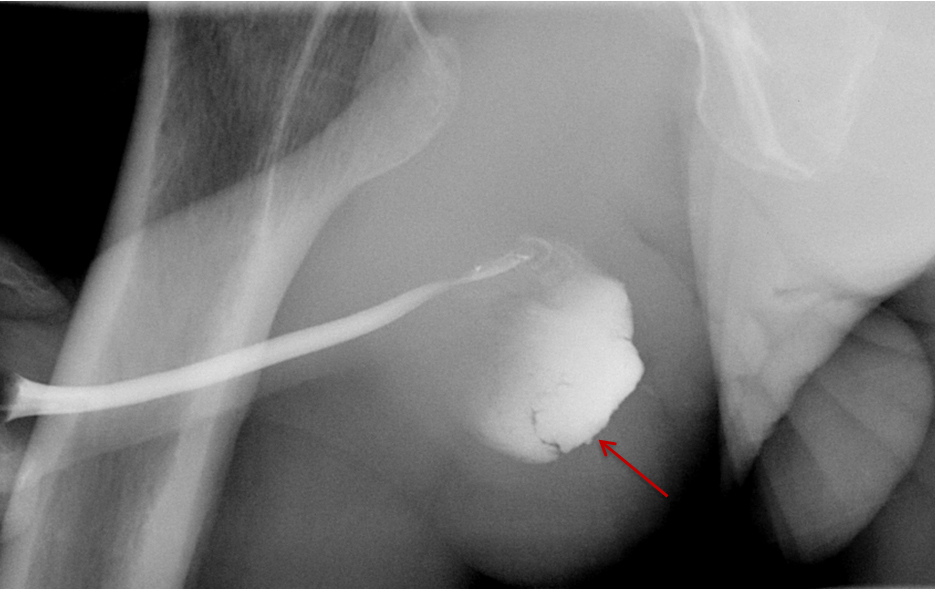
Penetrating urethral trauma usually results from knife or firearm injury and most commonly affects the anterior urethra. RUG examinations are indicated in patients with any penetrating penile injury, as up to 50% will have a urethral injury.12 Late complications of penetrating urethral trauma include periurethral abscess formation, post-traumatic stricture, and fistula formation.12,20 RUG will demonstrate contrast extravasation at the site of urethral injury (Figure 6). Extravasated contrast may be confused with urethral diverticulum when blunt or penetrating urethral injuries are being evaluated. In such scenarios, voiding images are particularly helpful as persistent contrast or a change in morphology is suggestive of urethral injury.
In the setting of complete urethral transection, retrograde instilled contrast will extravasate at the site of injury with non-opacification of the urethra proximal to the site of injury (Figures 6, 7).
After debridement of the urethral edges, urethral transections can be directly anastomosed over a Foley catheter (Figure 6).20 Long defects (> 2 cm) in penetrating trauma should not be repaired emergently; instead, they should be reconstructed at a later date, with urinary diversion in the interim.20 Injuries secondary to gunshot trauma also should not be repaired emergently secondary to a potentially delayed thermal injury; they should similarly be repaired in a delayed fashion.
Post-Traumatic Urethral Stricture
Urethral strictures are defined as narrowing of the urethra resulting from scar tissue formation from collagen and fibroblast proliferation, ultimately causing obstruction of the lower urinary tract. Etiologies for urethral strictures are multifactorial. The vast majority are secondary to traumatic, iatrogenic (indwelling catheter, cystoscopy, prior hypospadias correction, prostate surgeries), and idiopathic etiologies that encompass approximately 75% of all cases.21,22,23,24
Strictures manifest as narrowing of the contrast column on RUGs. Post-traumatic strictures may be single or multifocal and are typically variable in length (Figure 8). Most strictures involve the anterior urethra, with the bulbous segment most commonly affected.24,25,26,27 Posterior urethral strictures or stenoses are more prevalent in pelvic fractures, post-radiation treatment, or prior prostate surgery.26,27 During complete obstruction, retrograde instilled contrast will not be observed opacifying the segment of urethra more proximal to the stricture. In these cases, the urethra proximal to the obstruction may be evaluated with a combined antegrade and retrograde urethrogram (Figure 8).
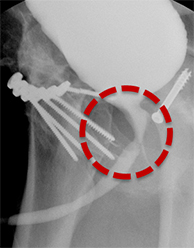
Treatment options for post-traumatic strictures include urethroplasty, urethral dilatation, urethrotomy, or suprapubic catheter placement. 24,25,26 Post-urethroplasty RUGs demonstrate improved caliber of the affected segment compared to preoperative films (Figure 9). In the immediate postoperative setting, a voiding cystourethrogram (VCUG) should be performed and careful attention should be paid to assessing for evidence of a leak. Urethral stents may be visualized as a radiopaque tubular device over the expected course of the urethra (Figure 9). However, these are no longer used due to complications of long-term patency secondary to intra-stent fibrosis.
Urethral Fistula
Urethral fistulas are most commonly acquired. Acquired causes include trauma, prior surgeries (up to 10% of complex hypospadias repair), prior infection, and post-radiation treatment-related changes. 28,29 While fistulas have been reported to be spontaneous in the setting of diabetes, they remain quite rare.28 Common acquired subtypes of urethral fistulas include urethrorectal and urethrocutaneous fistulas. Rarely, a urethroscrotal diverticulum may form.30 Urethrocavernous fistulas have been reported in penile fractures.31
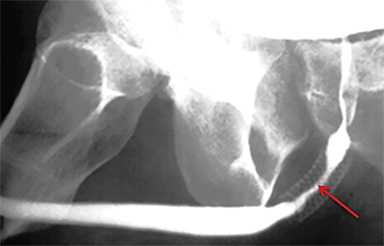
RUGs demonstrate an opacified fistulous tract extending from the urethra to the communicating organ. In cases of urethrorectal fistulas, the rectum will appear as an opacified structure with transverse folds distinct from the urinary bladder (Figure 10). Urethrocutaneous fistulas will demonstrate contrast extending to the skin surface; this may be readily evident on physical examination. In cases of urethroscrotal diverticula, contrast will opacify and distend the sac (Figure 10).30
Urethral fistulas are generally treated with urethroplasties, which take down the fistula, close the urethra, and fix any associated obstruction.29 Postoperative VCUG examinations following urethroplasty demonstrate nonopacification of previously noted fistulous tracts. A small opacified urethral outpouching at the fistula site may be observed corresponding to a small stump.
Conclusion
Retrograde urethrography continues to represent a cost-effective tool that remains the gold-standard for initial diagnostic evaluation of acute male urethral trauma, as well as post-traumatic pathologies, including stricture and fistula formation. With recent advances and complexity of lower urinary-tract surgical procedures, appropriate technique and interpretation of these examinations remains a crucial part of the radiologist’s skillset.
References
- Eaton J, Richenberg J. Imaging of the urethra: Current status. Imaging. 2005; 17(2): 139-149.
- McGeady JB, Breyer BN. Current epidemiology of genitourinary trauma. Urol Clin North Am. 2013;40(3):323-334.
- Van Den Driesen M, Kennedy M, Page P, et al. Emergency trauma urethrogram. TRM05.04 EMERGENCY TRAUMA URETHROGRAM. The Royal Melbourne Hospital. Trauma Service Guidelines. Version 3.0 June 2015
- Ingram MD, Watson SG, Skippage PL, et al. Urethral injuries after pelvic trauma: Evaluation with urethrography. RadioGraphics. 2008;28(6):1631-1643.
- Koraitim, et al. Pelvic fracture urethral injuries: The unresolved controversy. J Urol. 1999; 161(5):1433-1441.
- Maciejewski, C, Rourke K. Imaging of urethral stricture disease. Transl Androl Urol. 2015; 4(1): 2–9.
- Kohler T, Yadven M, Manvar A, et al. The length of the male urethra. Int Braz J Urol. 2008; 34(4): 451-456.
- Patel U, Rickards D, eds. Imaging and urodynamics of the lower urinary tract. London, England: Taylor & Francis, 2005; 115–121.
- Uehara DT, Eisner RF. Indications for retrograde cystourethrography in trauma. Ann Emerg Med. 1986; 15(3):270-272.
- Mundy AR, Andrich DE. Urethral strictures. BJU Int. 2011; 107(1): 6-26.
- Wessells H, Angermeier KW, et al. Male urethral stricture: AUA guideline. American Urological Association (AUA) Guideline. 2016.
- Kawashima A, Sandler CM, Wasserman NF, et al. Imaging of Urethral Disease: A Pictorial Review. Radiographics. 2004; 24:S195-S216.
- Sandler CM, Harris JH, Corriere JN, et al. Posterior urethral injuries after pelvic fracture. AJR Am J Roentenol. 1981; 137:1233-1237.
- Kong JPL, Bultitude MF, Royce P, et al. Lower urinary tract injuries following blunt trauma: a review of contemporary management. Rev Urol. 2011;13(3):119–130.
- Ingram MD, Watson SG, Skippage PL, et al. Urethral injuries after pelvic trauma: Evaluation with urethrography. RadioGraphics. 2008; 28(6):1631-1643.
- Djakovic N, et al. Guidelines on Urological Trauma. European Association of Urology: 2009
- Andrich DE, Day AC, Mundy AR. Proposed mechanisms of lower urinary tract injury in fractures of the pelvic ring. BJU Int. 2007;100(3):567–573.
- Durrant JJ, Ramasamy A, Salmon MS, et al. Pelvic fracture-related urethral and bladder injury. J R Army Med Corps. 2013;159: i32-i39.
- Rosenstein DI, Alsikafi NF. Diagnosis and classification of urethral injuries. Urol Clin North Am. 2006;33(1):73-85.
- Morey AF, Hernandez J, McAninch JW, et al. Reconstructive surgery for trauma of the lower urinary tract. Urol Clin North Am. 1999;26(1):49-60.
- Sievert KD, Selent-Stier C, Wiedemann J, et al. Introducing a large animal model to create urethral stricture similar to human stricture disease: a comparative experimental microscopic study. J Urol. 2012;187(3):1101–1109.
- Anger JT, Buckley JC, Santucci RA, et al. Trends in stricture management among male Medicare beneficiaries: underuse of urethroplasty? Urology. 2011;77(2):481–485.
- Mundy AR. Management of urethral strictures. Postgraduate Medical Journal. 2006;82(970):489-493.
- Hampson LA, McAninch JW, Bryer BN, et al. Male urethral strictures and their management. Nat Rev Urol. 2014;11(1):43-50.
- Felton A, Morey, AF, Aviles R, et al. Anterior urethral strictures: Etiology and characteristics. Urology. 65(6) 2005:1055-1058.
- Trischlet S, Roosen A, Fullhouse C, et al. Urethral stricture: Etiology, investigation and treatments. Disch Arztebl Intl. 2013; 110(13): 220-226.
- Yosif D. MyPACS.net Teaching File #36831558. Accessed 10/13/2017.
- Amukele SA, Stock JA, Hanna MK, et al. Management and outcome of complex hypospadias repairs. J Urol. 2005; 174(4 Pt 2):1540-1542.
- Akkoc A, Metin J. Spontaneous ventral urethral fistula in a young healthy man and a modified surgical technique of urethral fistula repair. Can Urol Assoc J. 2012;6(6):E280-E282.
- Parlak S, Okay AE. Urethroscrotal fistula: A rare cause of scrotal swelling. Pol J Radiol. 2016; 81:438-440.
- Palaniswamy R, Rao MS, Bapna BC, et al. Urethro-cavernous fistula from blunt penile trauma. J Trauma. 1981;21(3):242-243.
- Levin TL, Han B, Little BP. Congenital anomalies of the male urethra. Ped Radiol. 2007;37(9):851-862.
Citation
P H, T P, H P, C C, ME S, M M, O A.Post-traumatic Retrograde Urethrography: A Review of Acute Findings and Chronic Complications. Appl Radiol. 2020; (1):24-31.
January 23, 2020