Photon Counting CT Shows Hepatic Enhancement Using Gadoxetate Disodium in Phantom Study
Images
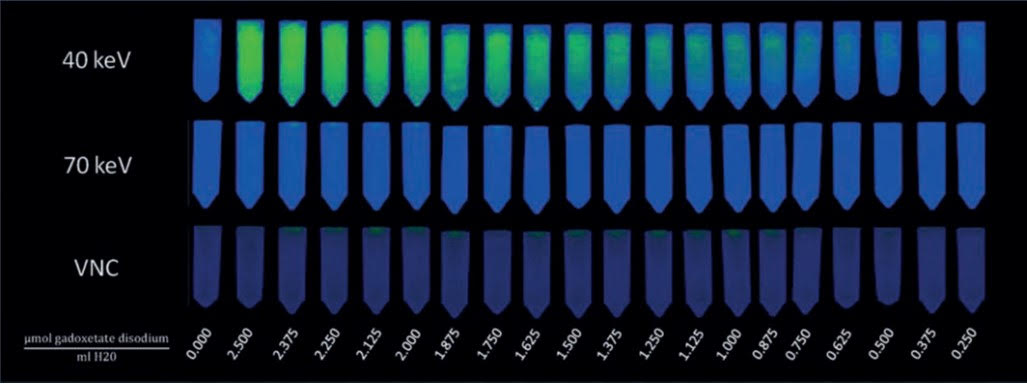
Research published in the American Journal of Roentgenology (AJR) reports photon-counting detector CT (PCD CT) yielded meaningful attenuation increases for gadoxetate disodium at considerably lower doses than previously documented for CT of gadolinium-based contrast agents (GBCAs)—albeit too high for clinical use at approximately eight-times greater than clinical doses.
“Combining gadoxetate disodium and PCD CT could theoretically allow appreciable hepatic enhancement at a 200-μmol/kg dose,” wrote lead investigator Stephan Rau, MD, from the department of diagnostic and interventional radiology at Germany’s University Medical Center Freiburg. “Such effect was not observed for the clinically approved 25- μmol/kg dose.”
Rau and colleagues prepared a series of solutions containing diluted gadoxetate disodium (concentrations of 0.250-2.5 μmol/ml, corresponding with doses of 25-200 μmol/kg, respectively). Along with deionized water, their solutions were evaluated in an anthropomorphic abdominal phantom using a clinical PCD-CT scanner; virtual monoenergetic images (VMI) at 40, 50, 60, and 70 keV, along with virtual noncontrast (VMC) images, were generated. After attaining attenuation measurements, the AJR authors combined a linear regression model of values with previously reported in vivo data to estimate hepatic enhancement and CNR across doses.
Ultimately, PCD CT with gadoxetate disodium yielded progressive increases in attenuation at increasing concentrations. Estimated in vivo hepatic enhancement at 40 keV was 4.9 HU for the agent’s clinically approved dose of 25 μmol/kg, versus 19.9 HU for 100 μmol/kg and 30.8 HU for 200 μmol/kg.
“Additional research exploiting PCD-CT technology could seek to reduce further doses required for sufficient visualization into a clinically feasible range, to potentially allow CT using a liver-specific agent,” the authors of this AJR accepted manuscript added.