Patent Awarded to UCLA for PET Radiopharmaceutical
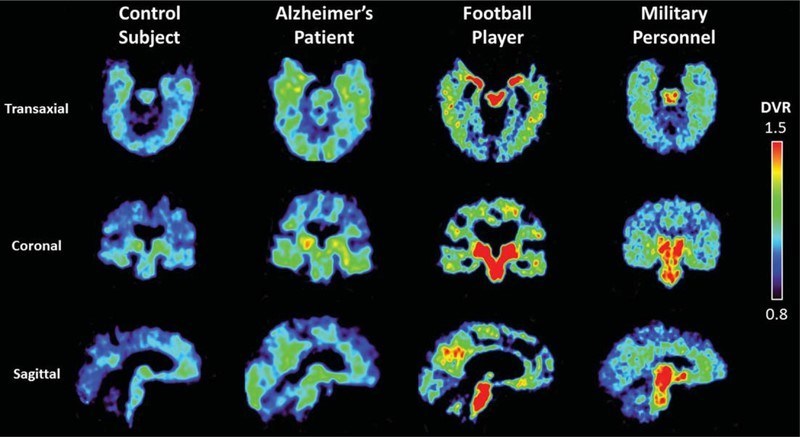 A US patent was awarded to UCLA for Flornaptitril, a PET scan imaging radiopharmaceutical that is licensed exclusively to CereMark. Clinical studies show that Flornaptitril binds with both tau aggregates and beta amyloid plaque in the brain to enhance PET scan imaging of these pathological proteins and the distribution patterns which are determinative in diagnosing between Chronic Traumatic Encephalopathy (CTE)and Alzheimer's Disease (AD). Through two phases of clinical study, several hundred subjects with cognitive impairment from either traumatic brain injuries or disease have been safely studied with Flornaptitril.
A US patent was awarded to UCLA for Flornaptitril, a PET scan imaging radiopharmaceutical that is licensed exclusively to CereMark. Clinical studies show that Flornaptitril binds with both tau aggregates and beta amyloid plaque in the brain to enhance PET scan imaging of these pathological proteins and the distribution patterns which are determinative in diagnosing between Chronic Traumatic Encephalopathy (CTE)and Alzheimer's Disease (AD). Through two phases of clinical study, several hundred subjects with cognitive impairment from either traumatic brain injuries or disease have been safely studied with Flornaptitril.
"Neurodegenerative brain disorders are a growing concern for doctors, patients and families, with everyone wanting to find better ways to address the needs of those patients presenting with mild to severe cognitive decline. We are excited that research we are accomplishing with Flornaptitril could represent a significant breakthrough in both diagnosis and charting responses to treatments, and better management for neurodegenerative diseases," said Dr. Henry M. Chilton, CEO of CereMark.
Additionally, Dr. William C. Putnam, Chief Regulatory and Science Officer commented, "We are well positioned for continued success in the Phase 3 trial for Flornaptitril as no single PET imaging agent yet approved by FDA simultaneously detects both tau aggregates and beta amyloid plaque in neurodegeneration in a single scan. We are close to finalizing with FDA the protocol for our Phase 3 multi-site clinical trial for this truly unique PET imaging radiopharmaceutical."
CereMark Pharma, LLC is a developmental and clinical stage pharma company that focuses upon neurodegenerative diseases, including early diagnosis and management, as well as preventative, non-invasive management of chronic neurological impairment from events associated with traumatic brain injury.
CereMark's products are in various stages of nonclinical and clinical development and are not yet approved for human use.
Related Articles
Citation
. Patent Awarded to UCLA for PET Radiopharmaceutical. Appl Radiol.
May 3, 2021