Non-neoplastic Cystic Lesions of the Central Nervous System, Part 1: Developmental Cysts
Images

Editor’s note: This is the first part of a two-part series. The second part will appear in the September/October 2022 issue of Applied Radiology.
A variety of developmental and acquired intracranial cysts are frequently encountered on imaging. Knowledge of their imaging characteristics, localization, and clinical behavior improves diagnostic accuracy. In this first part of a two-part series, we review non-neoplastic, developmental cystic lesions, including prominent perivascular/Virchow-Robin spaces, epidermoid, dermoid, colloid cyst, arachnoid cyst, and Rathke cleft cysts. Epidemiologic and pathophysiologic features, clinical presentation, diagnosis, and radiologic characteristics for each will be discussed.
Prominent Perivascular (Virchow-Robin) Spaces
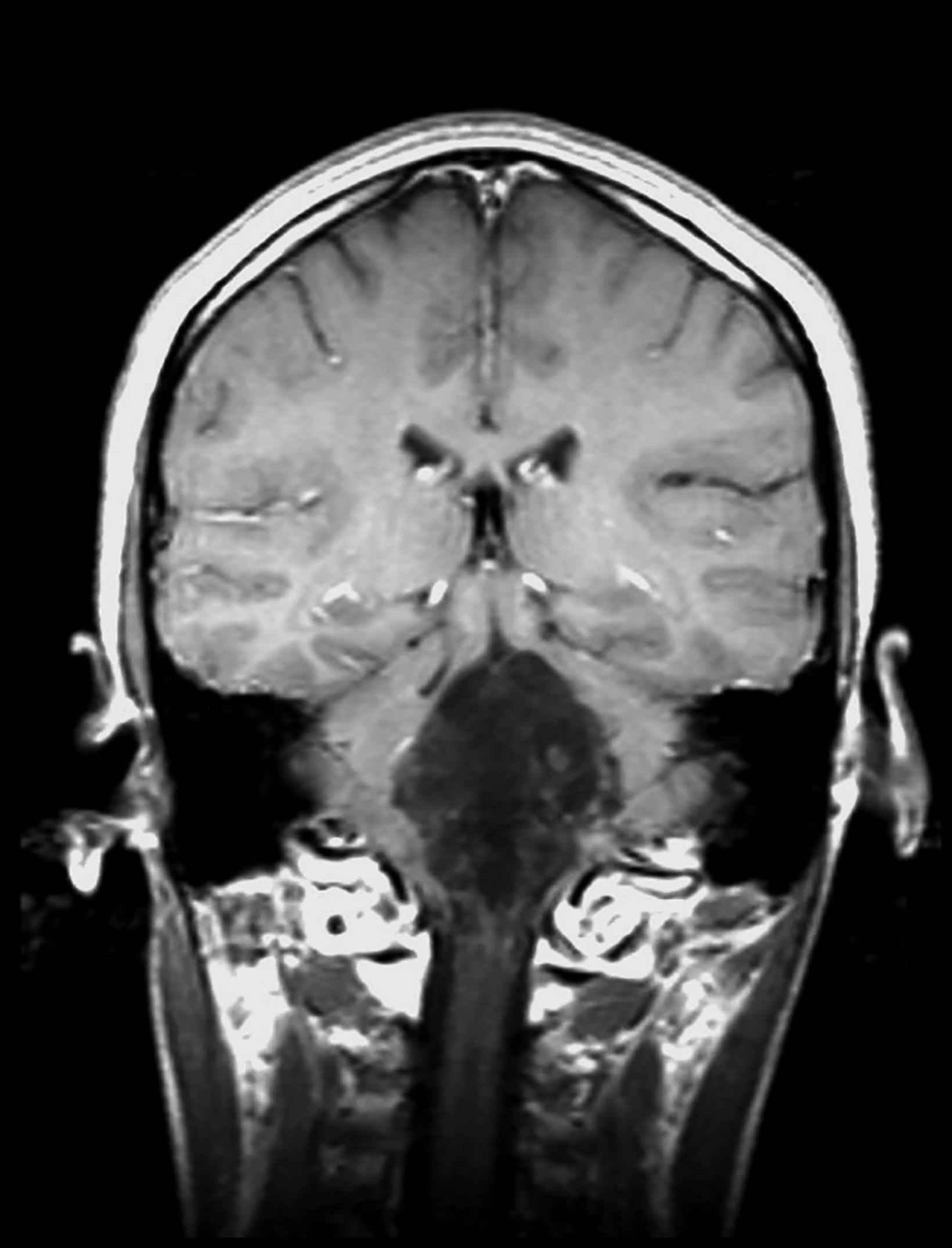
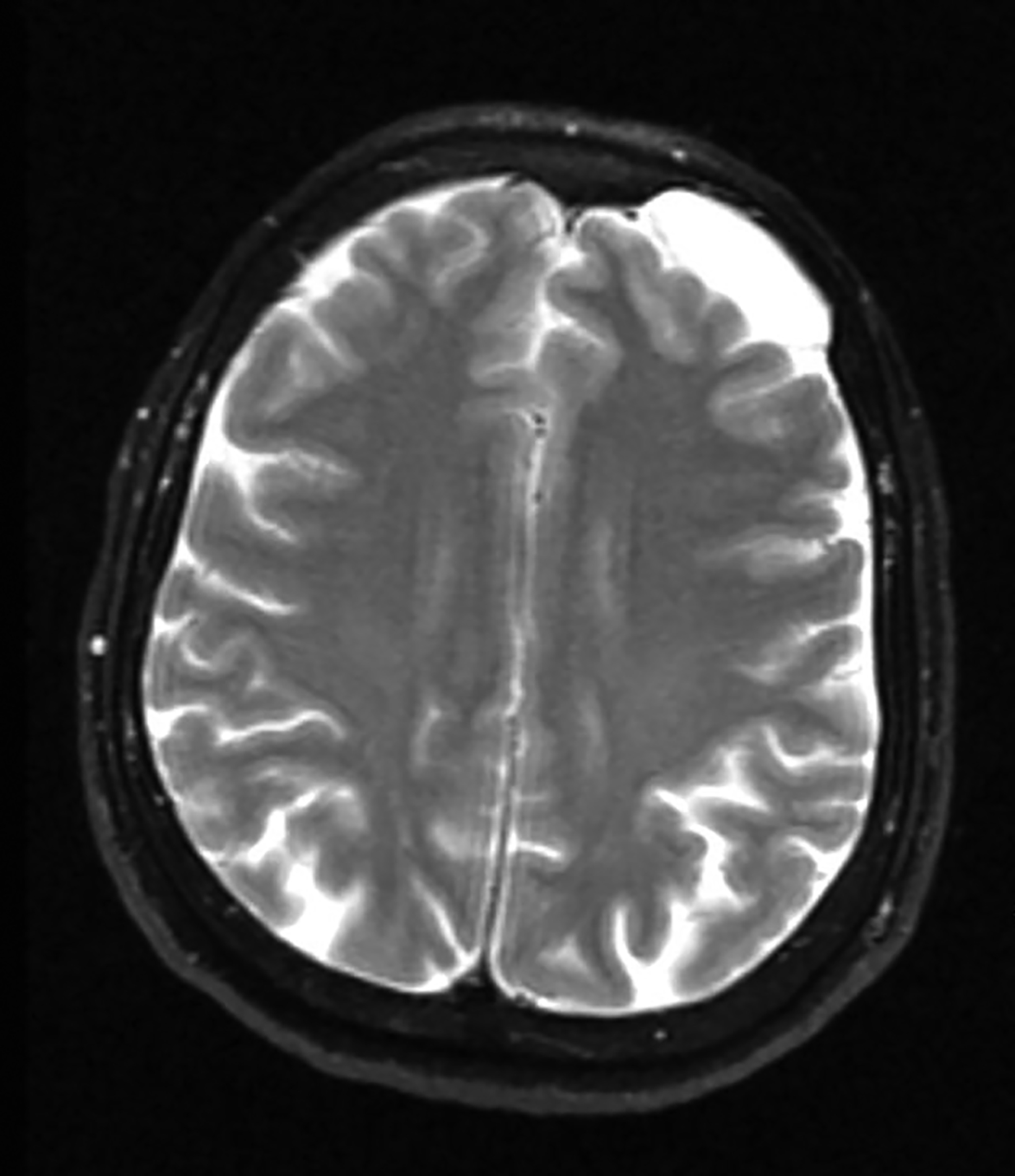
Perivascular spaces (PVS) are not true lesions. As the name implies, they are a result of blood vessels penetrating the brain parenchyma attaining a peripheral pial and arachnoid covering; thus, they contain variable amounts of cerebrospinal fluid (CSF) in a potential small subarachnoid space.1 These are not specific to one age group and the incidence has been reported to increase with age.1 Perivascular spaces are potential conduits for leptomeningeal spread of tumors and infections.2 Prominent or giant PVS exceeding 15 mm can be seen in the inferomedial temporal lobes, pons, and deep nuclei.1 Most large PVSs are found incidentally; however, they may be seen in patients suffering from headache and other neurological symptoms (Figure 1).
A knowledge of the imaging appearances and typical locations of these cyst-like structures should avoid raising unnecessary concerns. Magnetic resonance imaging (MRI) is the preferred modality to confirm giant PVS, but they can also be diagnosed with computed tomography (CT.) On both modalities these structures behave similarly to CSF and show no contrast enhancement in the wall.1,3
Epidermoid Cysts
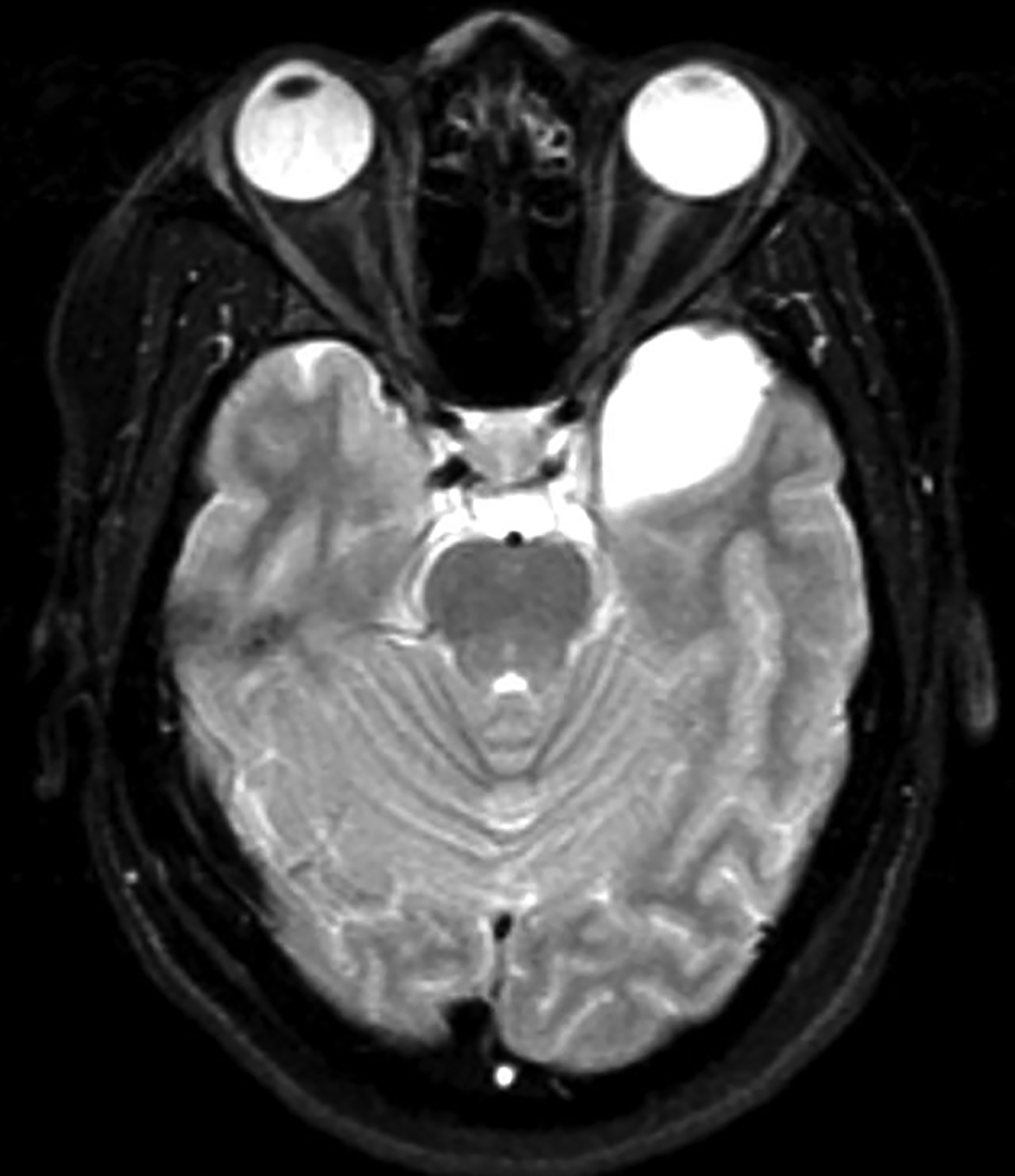
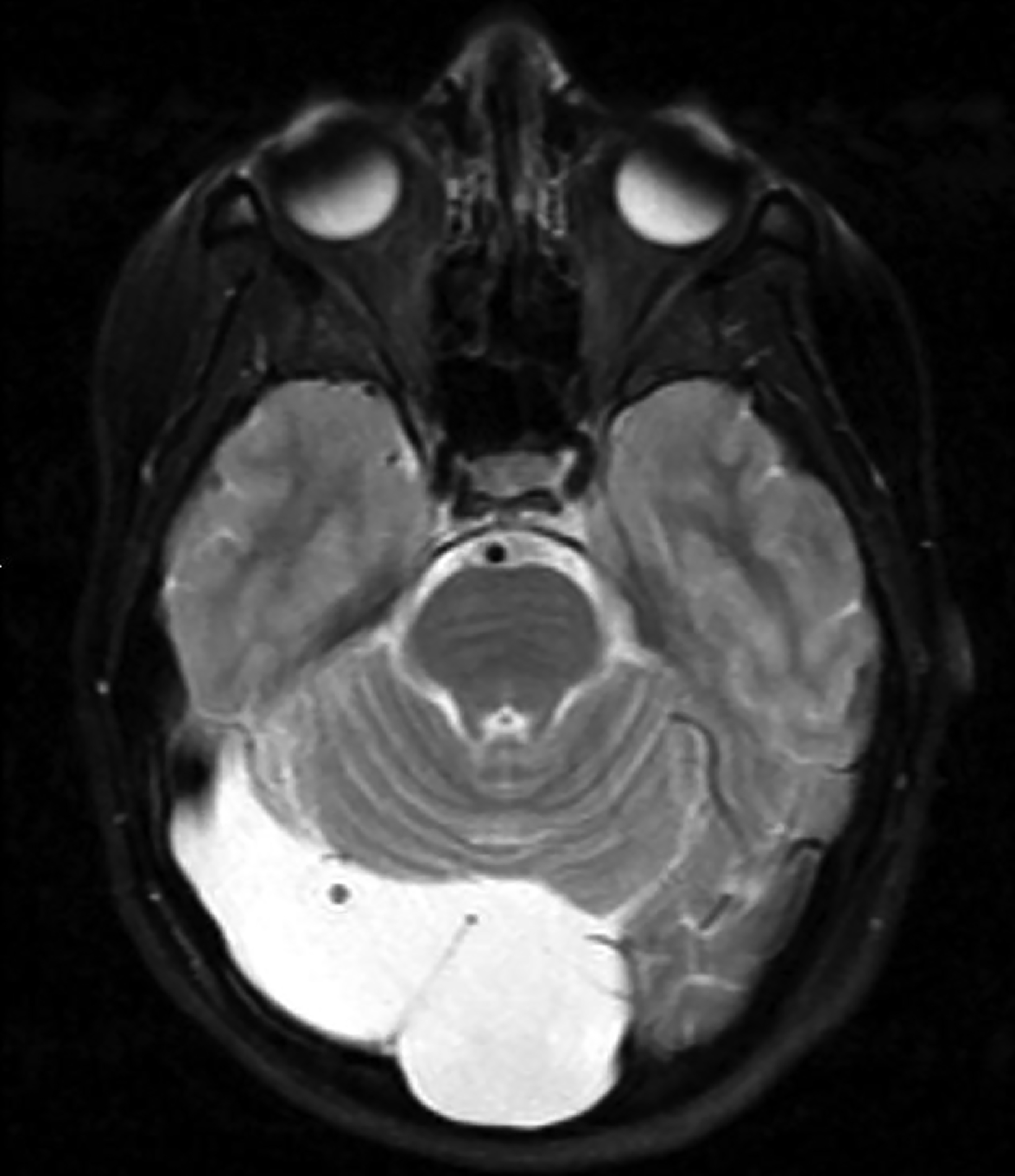
Epidermoid cysts account for approximately 1-2% of all intracranial tumors.4 Men and women are affected equally, with peak incidence in the third and fourth decades. They result from displacement of ectodermal elements during neural tube closure.5 These tumors are usually located in the parasellar region and cerebellopontine angle and less frequently in the suprasellar cistern, cerebral and cerebellar hemispheres. Epidermoids expand slowly with benign histopathologic features. Malignant transformation of epidermoid is extremely rare. Most patients become symptomatic during adulthood.4 Signs and symptoms generally result from mass effect on adjacent structures and include headache, cranial nerve deficits, and seizures.
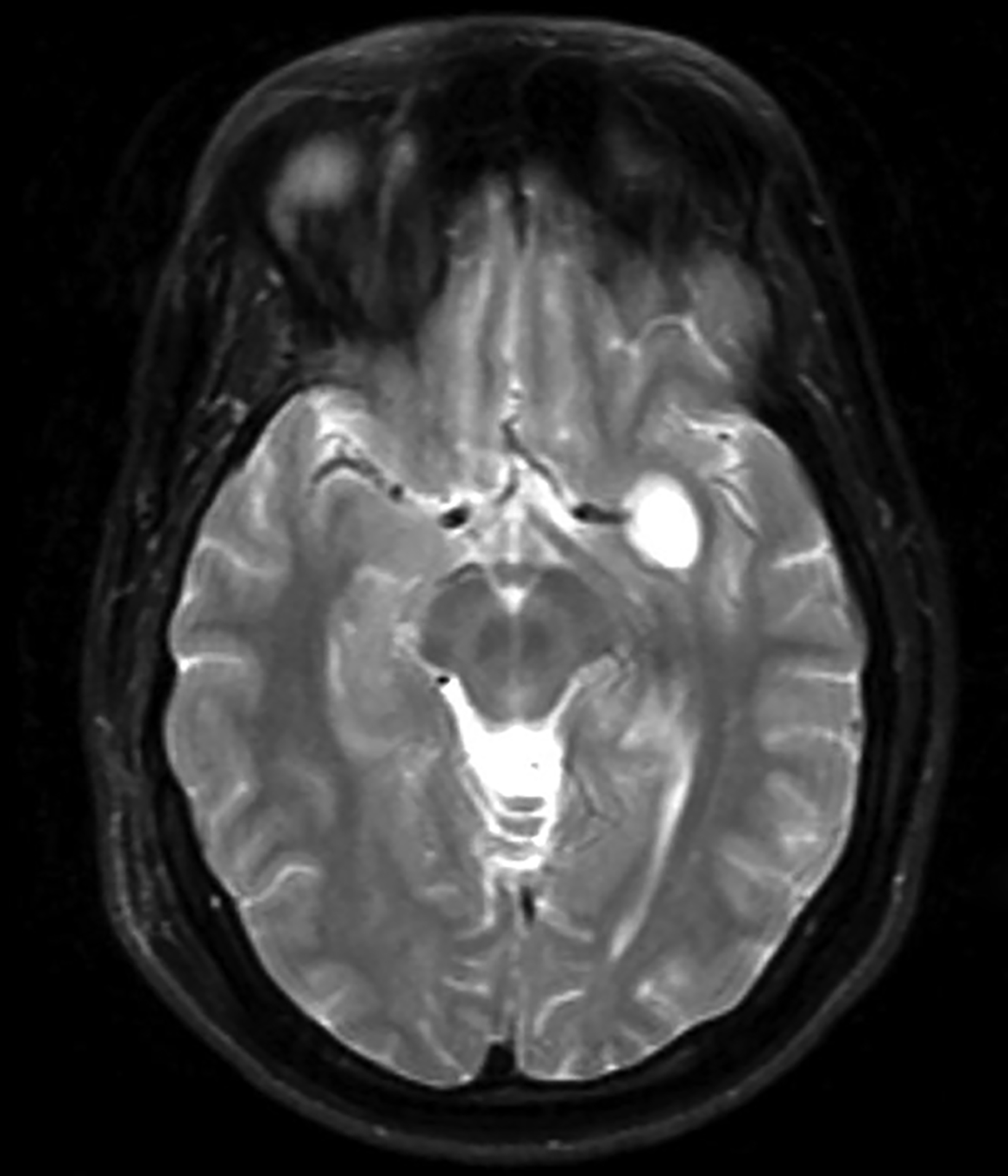
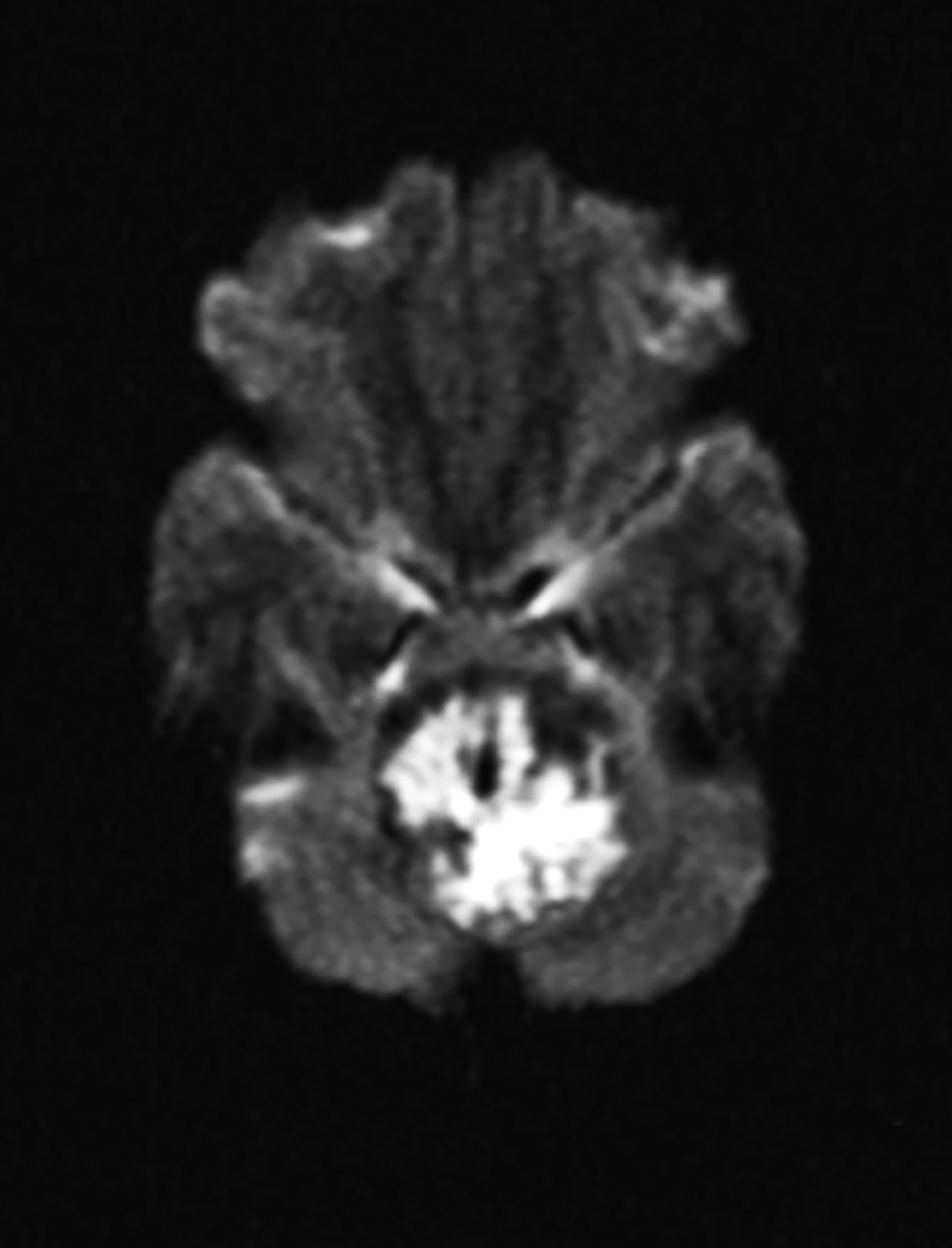
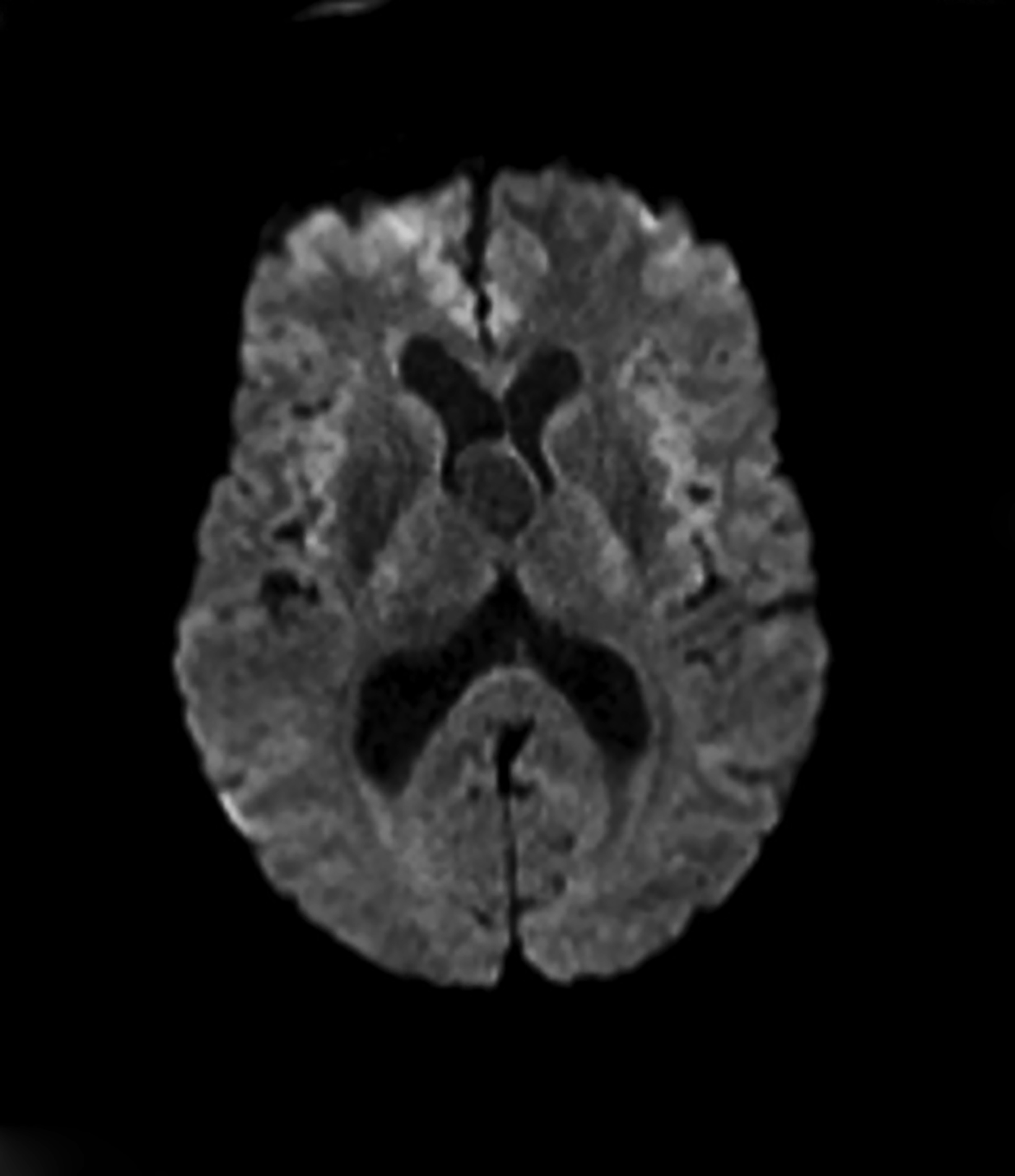
Extradural epidermoids are less frequent than intradural ones, usually occur in the temporal bones, and appear as well-defined lesions with scalloped margins. Epidermoids are hypodense on CT, similar to or slightly higher than CSF, with minimal to no peripheral enhancement. Up to 25% of epidermoids show calcification on CT. Findings on MRI depend on maturation of the cysts but generally epidermoids are hypointense on T1 images and hyperintense on T2 images.6 Distinguishing epidermoid from arachnoid cysts can be challenging with CT; MRI is more useful, as epidermoids are brighter than CSF on fluid attenuation inversion recovery (FLAIR) and, unlike arachnoid cysts, which demonstrate facilitated diffusion owing to their cystic nature, epidermoids typically have reduced diffusion on diffusion weighted imaging (DWI) with low apparent diffusion coefficient (ADC) values (Figure 2).
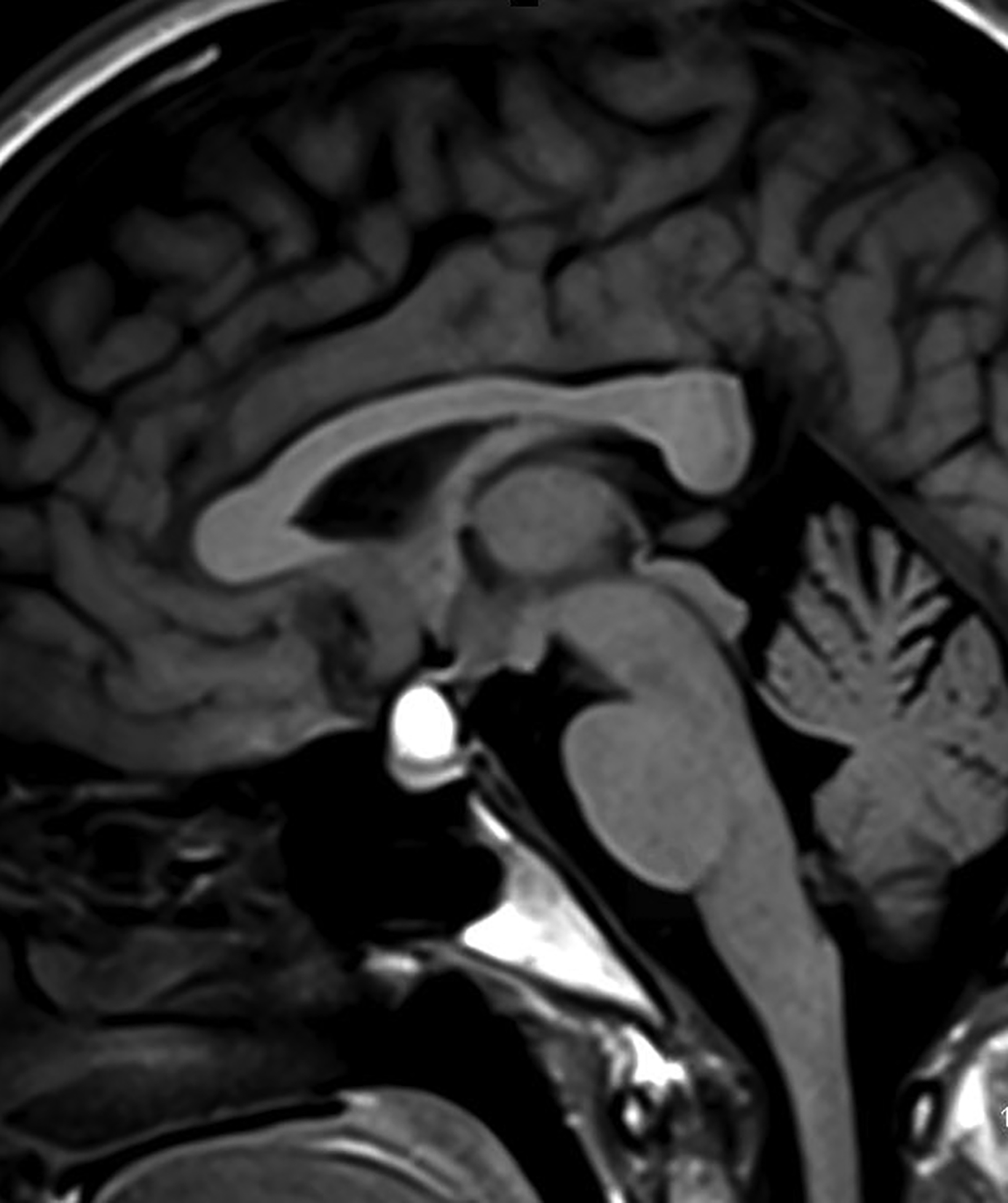
Dermoid Cysts
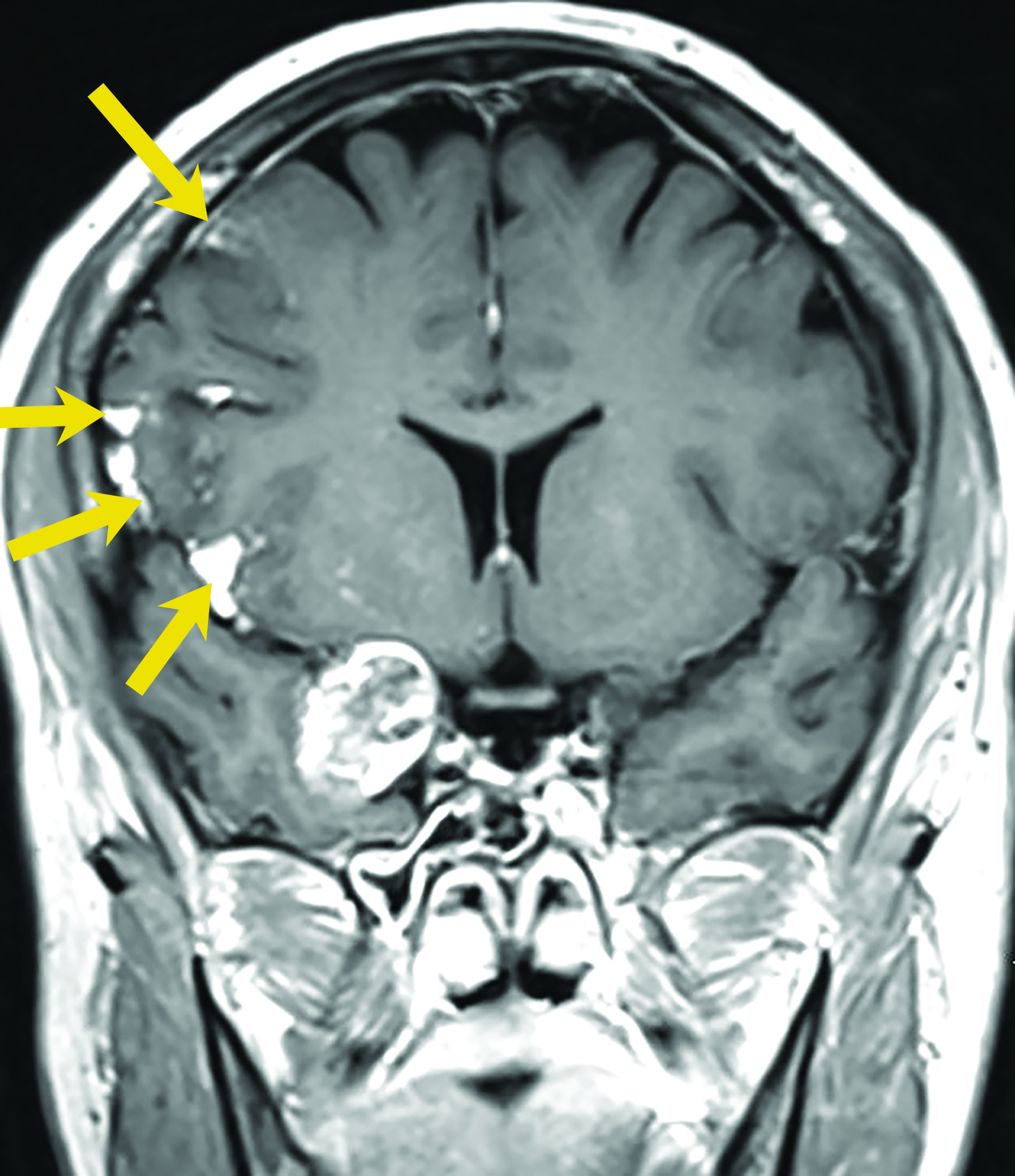
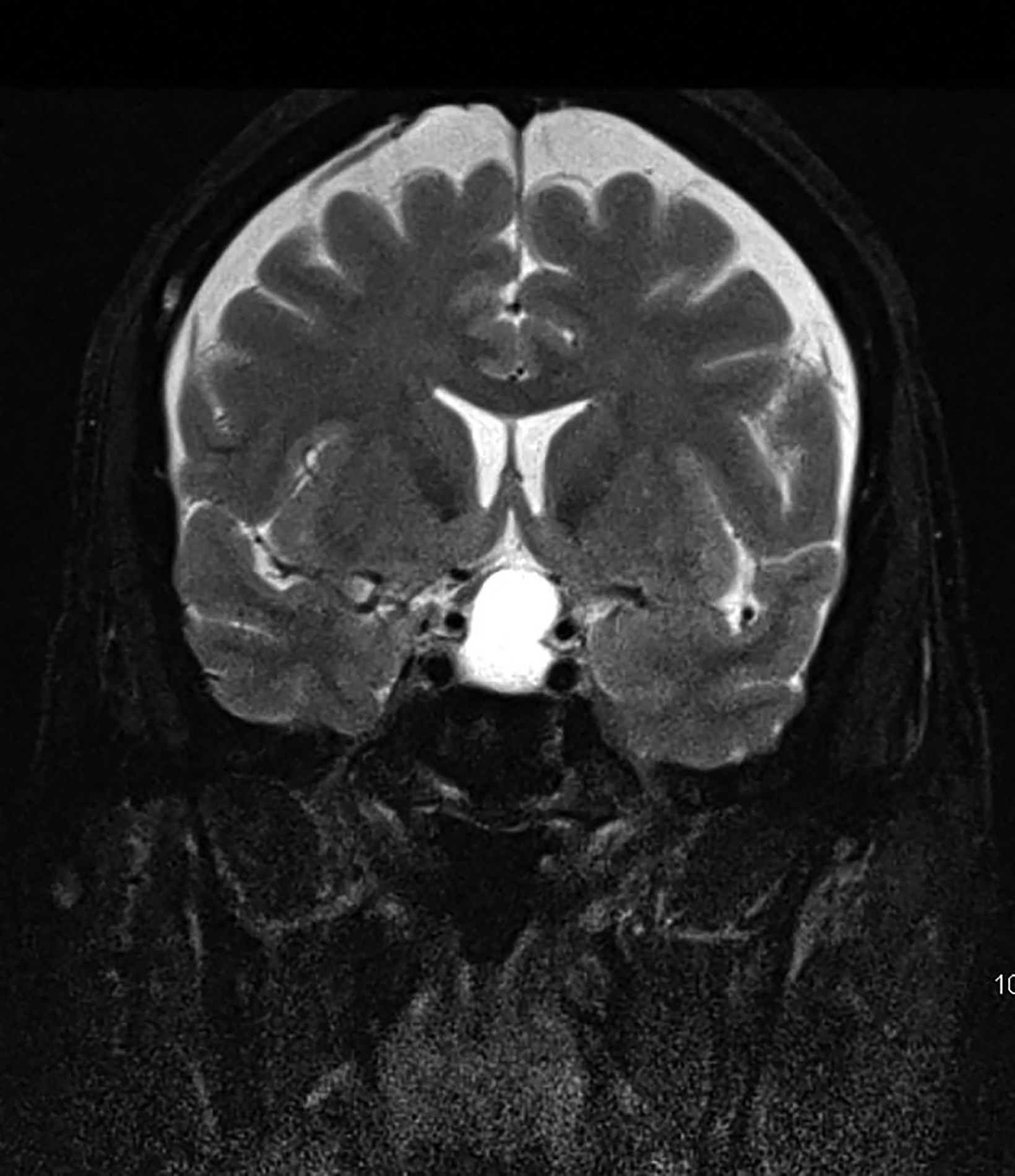
Intracranial dermoids are rare, accounting for 1% of all intracranial lesions.7 Men are affected more commonly than women, and patients are younger than those with epidermoids at presentation. Unlike epidermoids, these lesions tend to occur in the supratentorial midline or paramedian locations. The typical location for a dermoid is in the suprasellar cistern or in the temporosylvian region.7 Most patients with dermoids are asymptomatic. Symptoms usually result from mass effect on adjacent structures or chemical meningitis upon rupture of the cyst and leakage into the subarachnoid space.
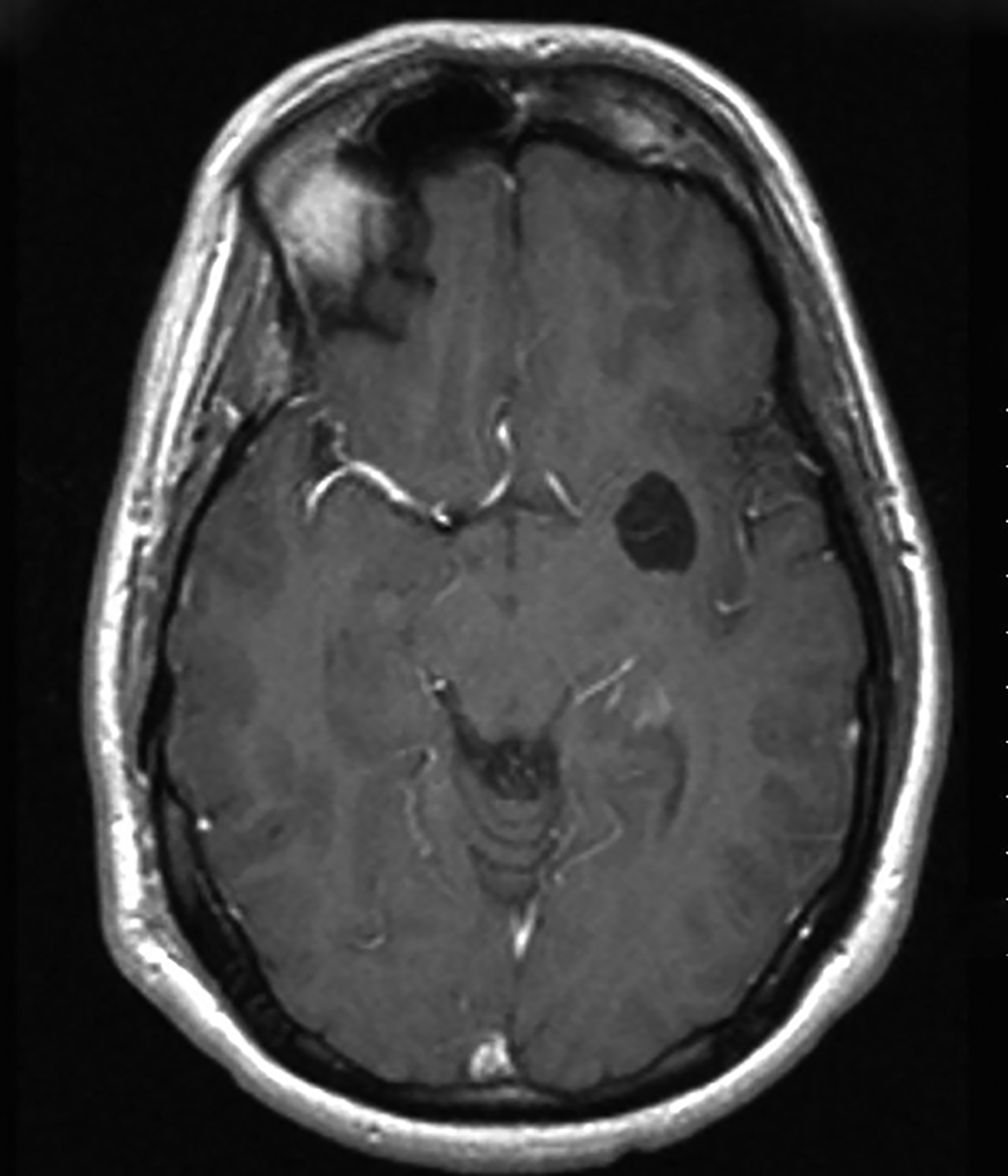
Dermoid cysts have characteristic imaging appearances. They present as well-defined, lobulated midline lesions on CT with fat density and calcification in the wall. Contrast enhancement is uncommon in dermoids. Lesions are hyperintense on T1 images with signal void that corresponds to calcification. Dermoids do not show enhancement with gadolinium on MRI. Dermoid signal varies from hypo- to hyperintense on T2 images (Figure 3). Classically, when a dermoid ruptures, fat droplets will appear scattered in the subarachnoid spaces and/or intraventricularly, best seen as hyperintense on T1 images; chemical meningitis can result in leptomeningeal enhancement.
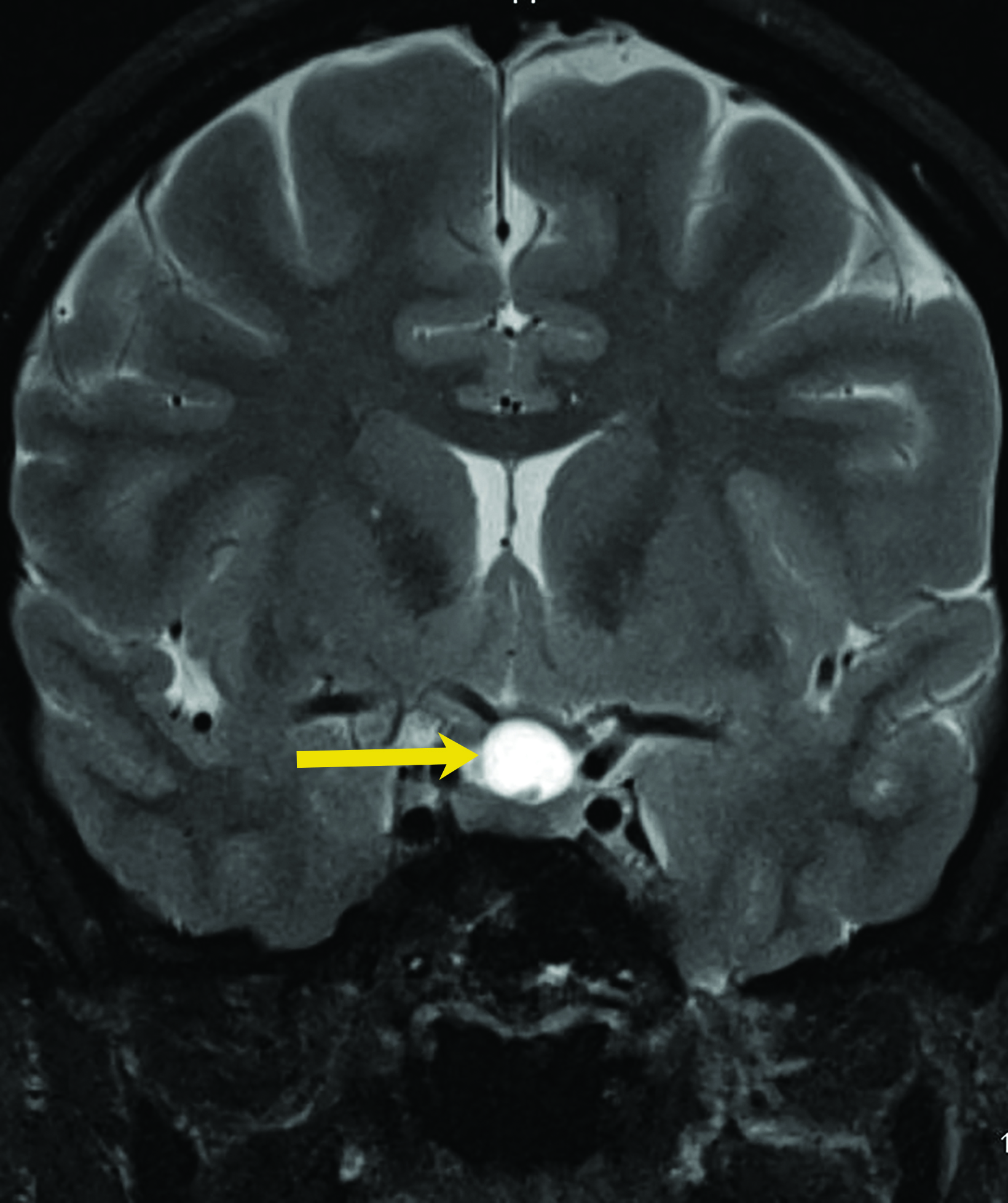
Colloid Cysts
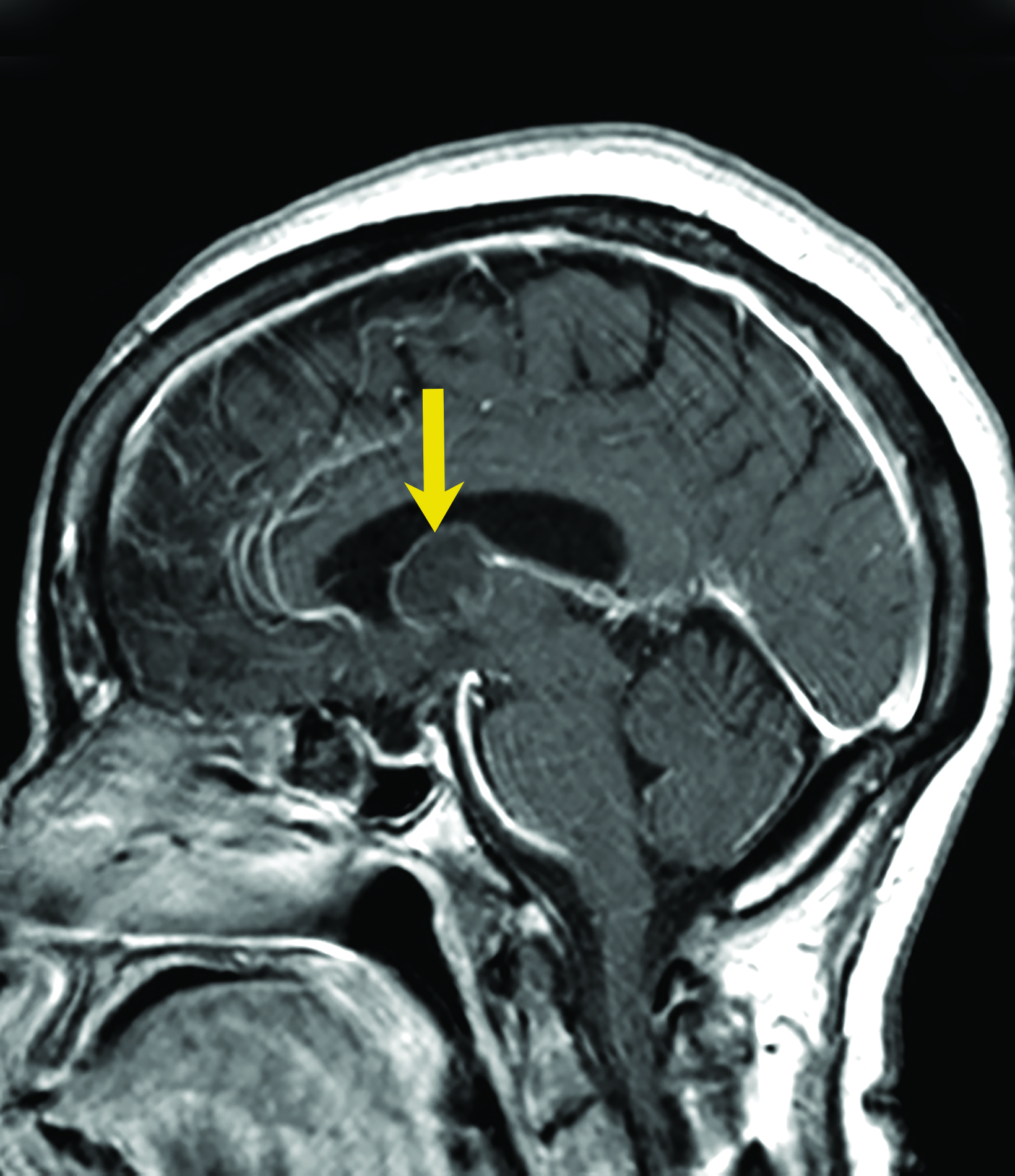
Colloid cysts are benign growths that occur primarily in the wall of the third ventricle. These cysts are the result of abnormal folding of the neuroepithelial paraphysis during development.8 Colloid cysts are lined by columnar epithelium that secretes mucin. They account for up to 2% of all primary brain tumors,8 typically present between the third and fifth decades of life, and are more common in men.9
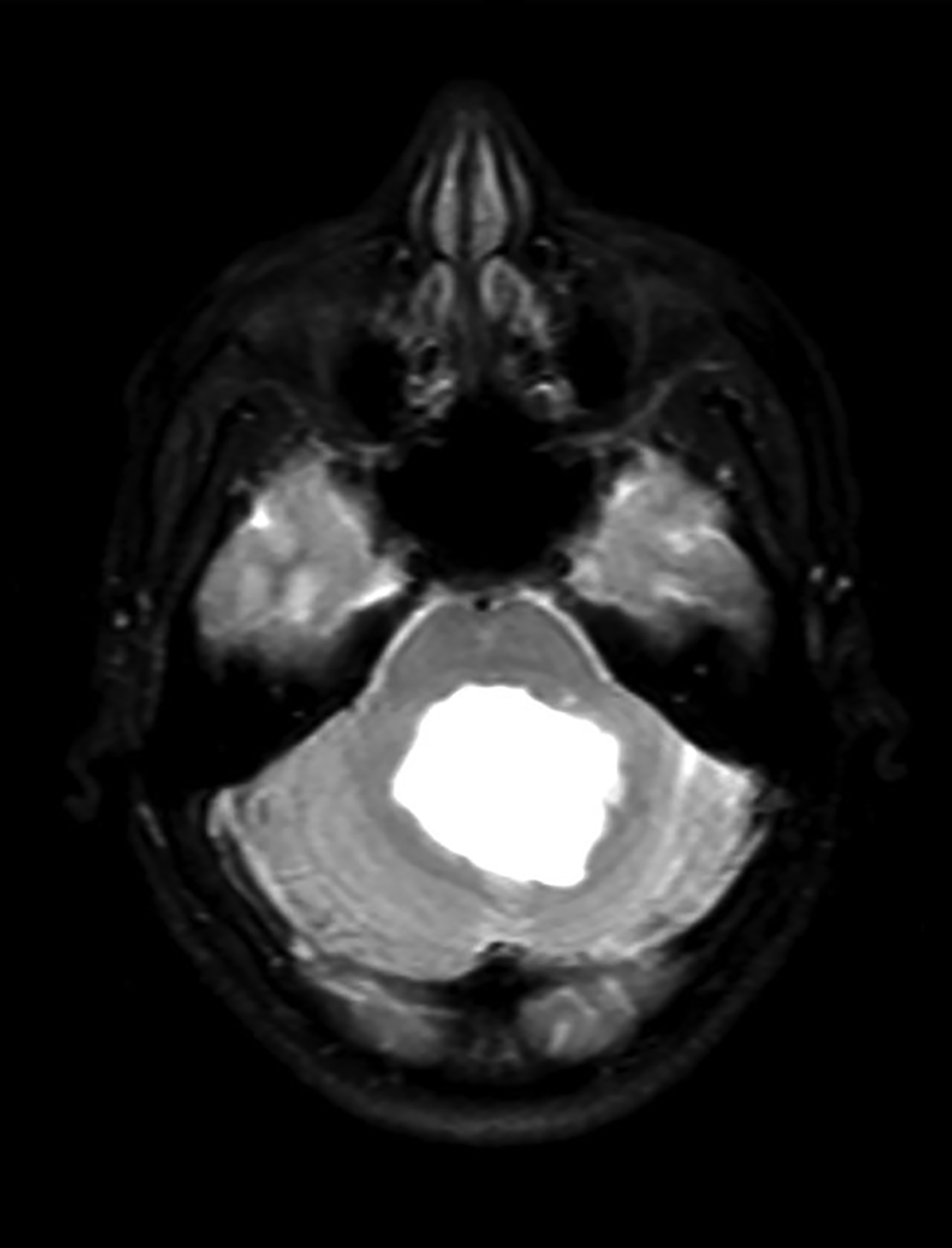
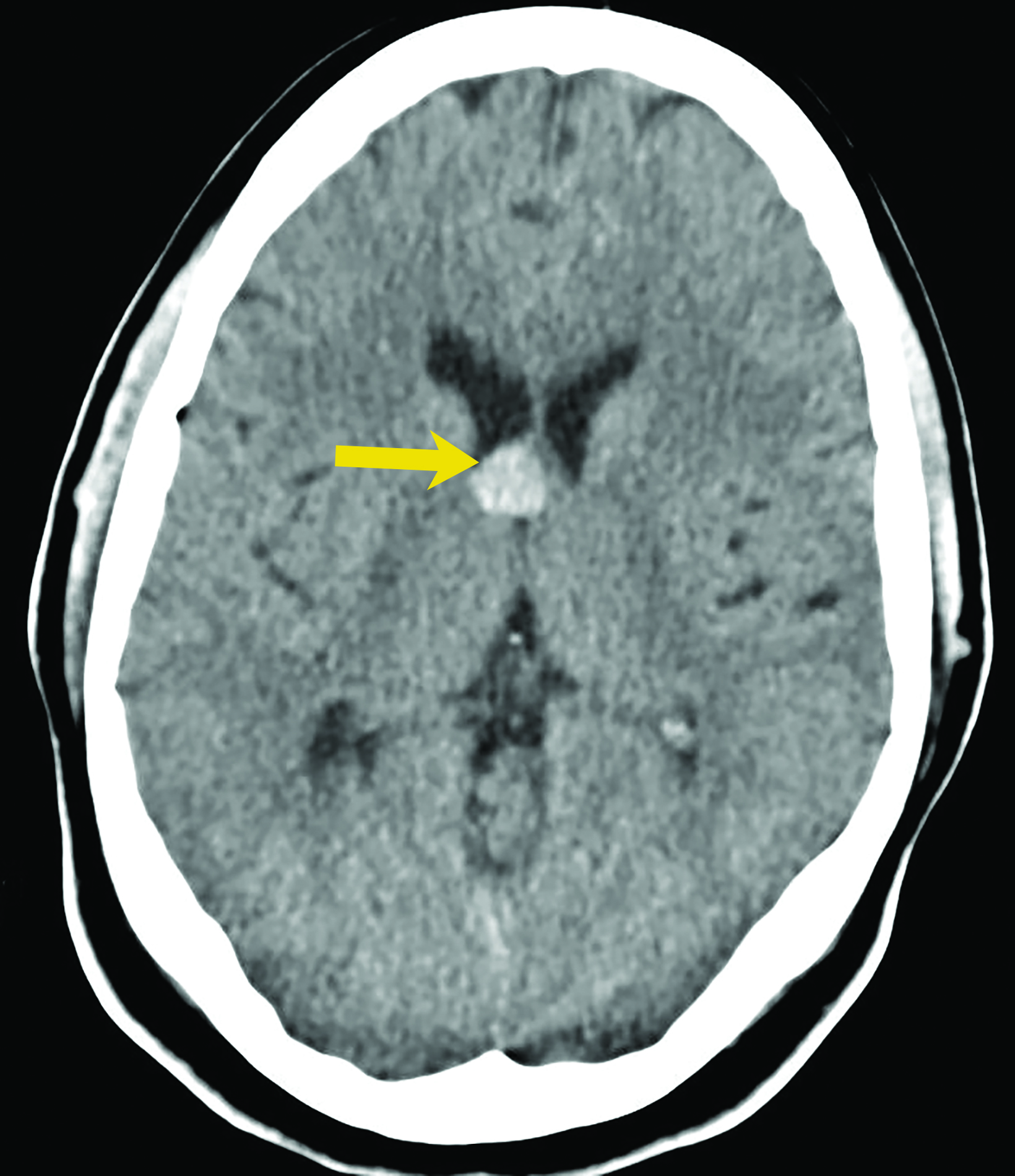
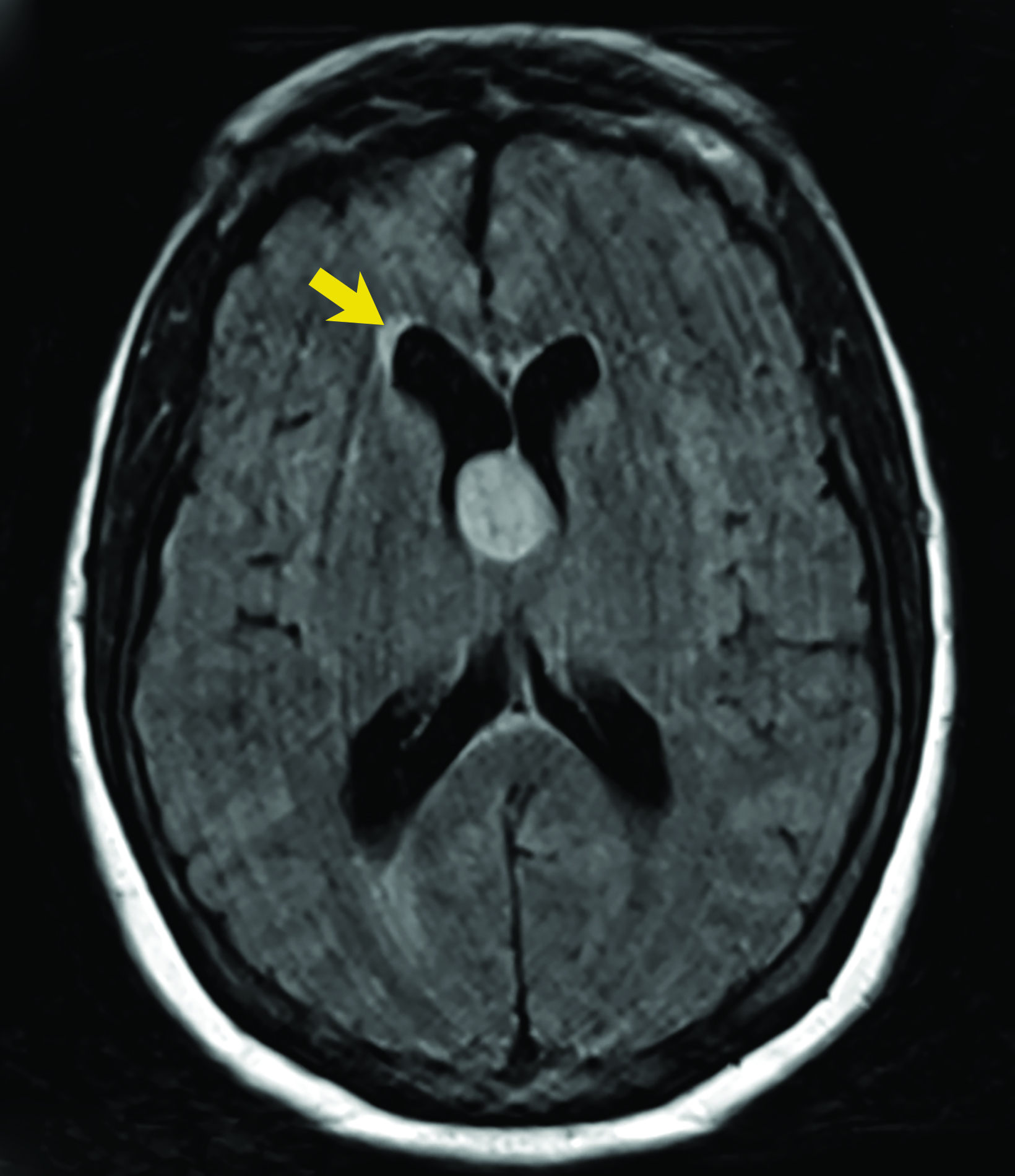
Asymptomatic colloid cysts are seen incidentally on imaging. Although benign, they can cause acute obstruction of the foramen of Monro, leading to hydrocephalus and herniation, especially after acute intracystic hemorrhage (Figure 4). Symptomatic colloid cysts present with severe headaches and vomiting and may also cause seizures, especially in cases diagnosed in the first two decades of life.10 Patients might also present with intermittent headaches that tend to improve when supine.9
Colloid cysts can be seen on CT and MRI. On CT, they are easily seen as well-defined, hyperdense, round lesions, but they can also be subtle when they are small hypodense or isodense lesions (Figure 4). Their intensity on MRI is dependent on their contents. Colloid cysts typically appear hyperintense on T1 and hypointense to isointense on T2 when compared to brain tissue. Colloid cysts may not be well seen on FLAIR sequences because of the dark signal of the cyst blending with adjacent CSF.11 The DWI signal also varies with the contents of the cyst (Figure 4).
Arachnoid Cysts
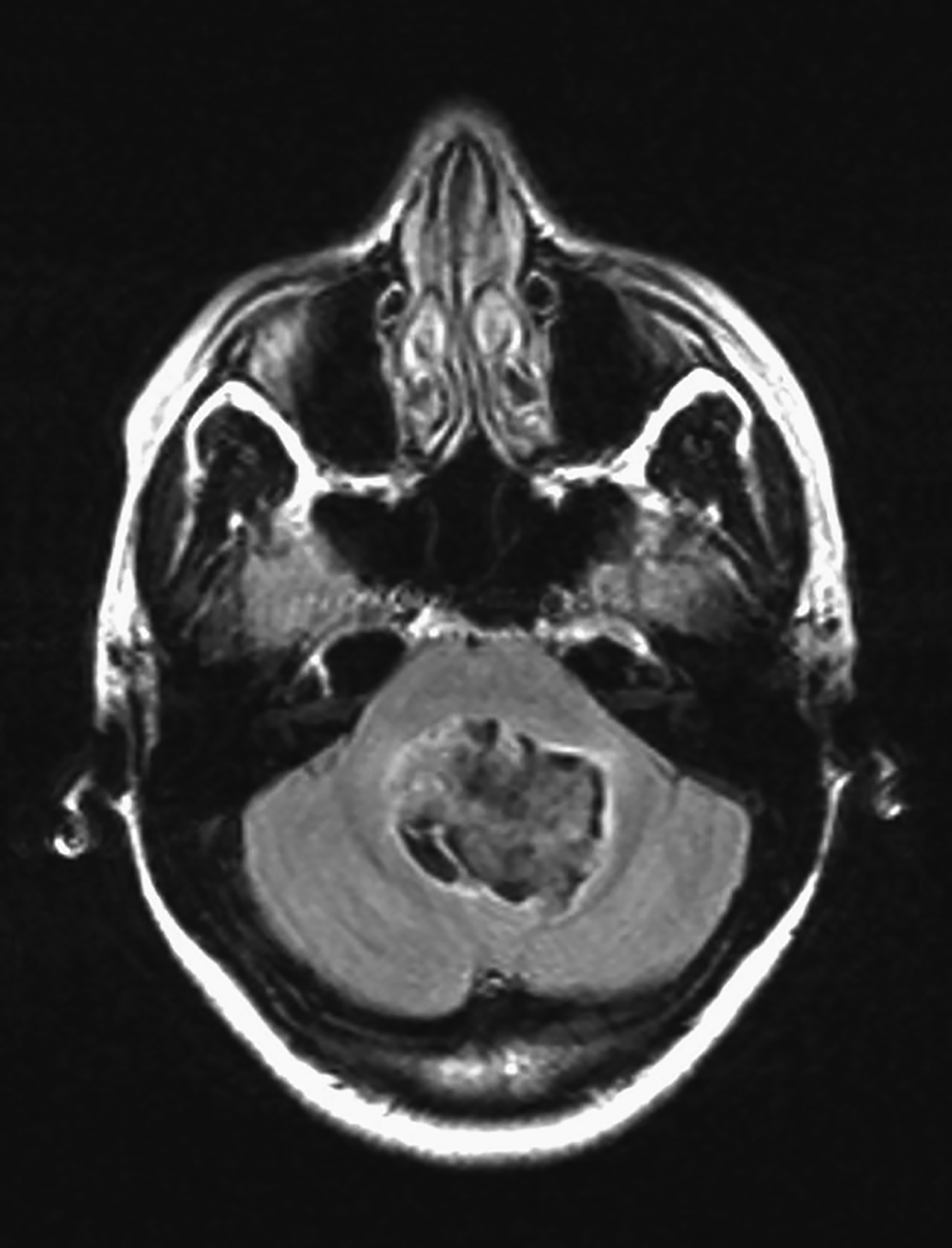
Arachnoid cysts are non-neoplastic intracranial lesions that contain cerebrospinal fluid (Figure 5). These cysts are lined by arachnoid cells and have a prevalence of 1.4% in the adult population. The prevalence of these cysts is slightly higher, 2.6%, in the pediatric population. While most arachnoid cysts are considered developmental, some may develop subsequent to head injury and infection.12 Arachnoid cysts have been reported in various locations of the brain, including but not limited to the cerebral convexities, posterior fossa and, most commonly, the middle cranial fossa.13
Most patients with arachnoid cysts are asymptomatic. Symptoms may arise depending on the location and size of the cyst and in the event of complications such as acute intracystic hemorrhage. Symptoms most often consist of headache but may also include seizures or focal neurological deficits.14
Arachnoid cysts are seen on CT and MRI as well-defined, extra-axial entities that are isodense/isointense to CSF.10 Fluid-fluid level can be seen in hemorrhagic cysts. Arachnoid cysts typically do not enhance.15
Rathke Cleft Cysts
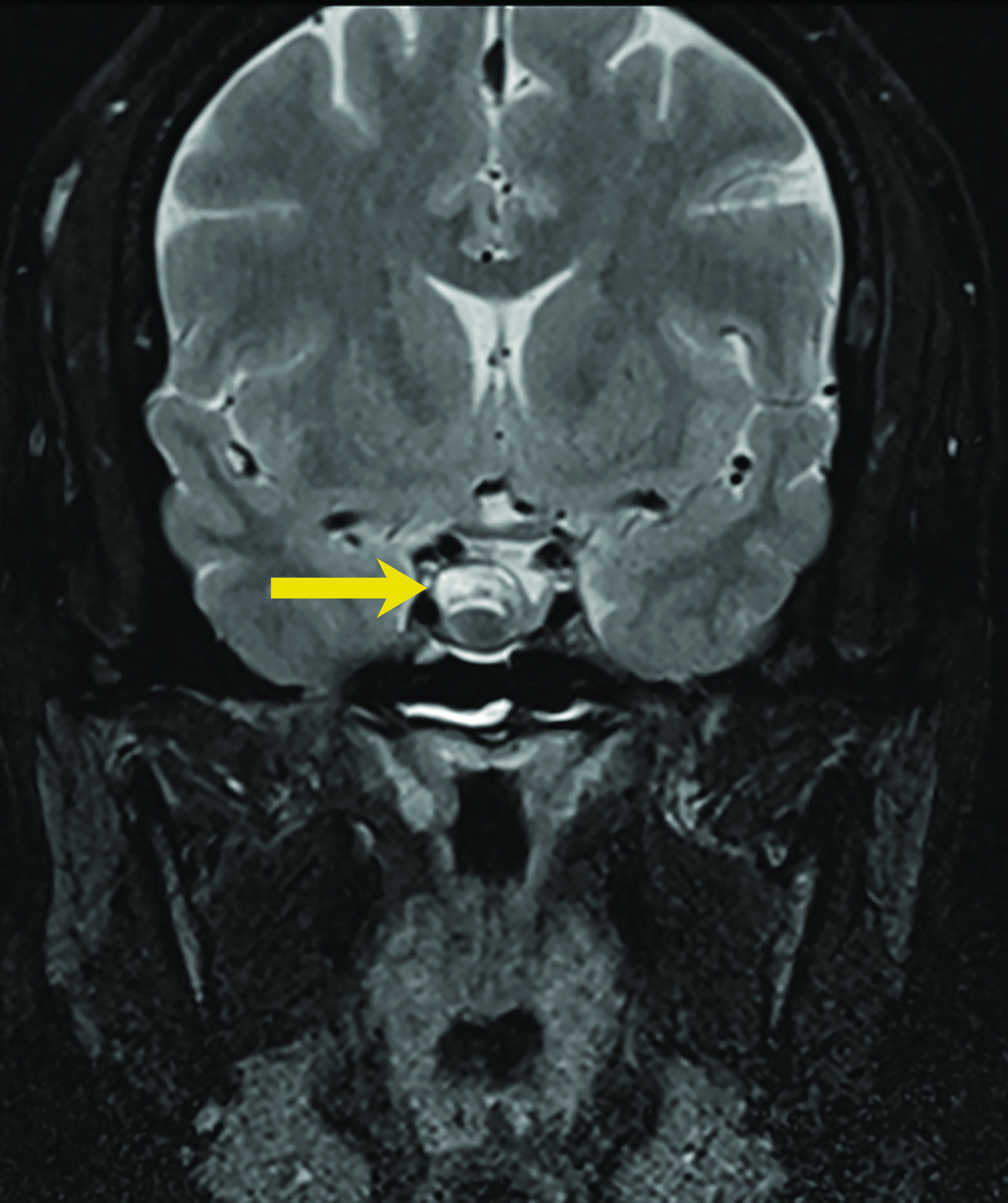
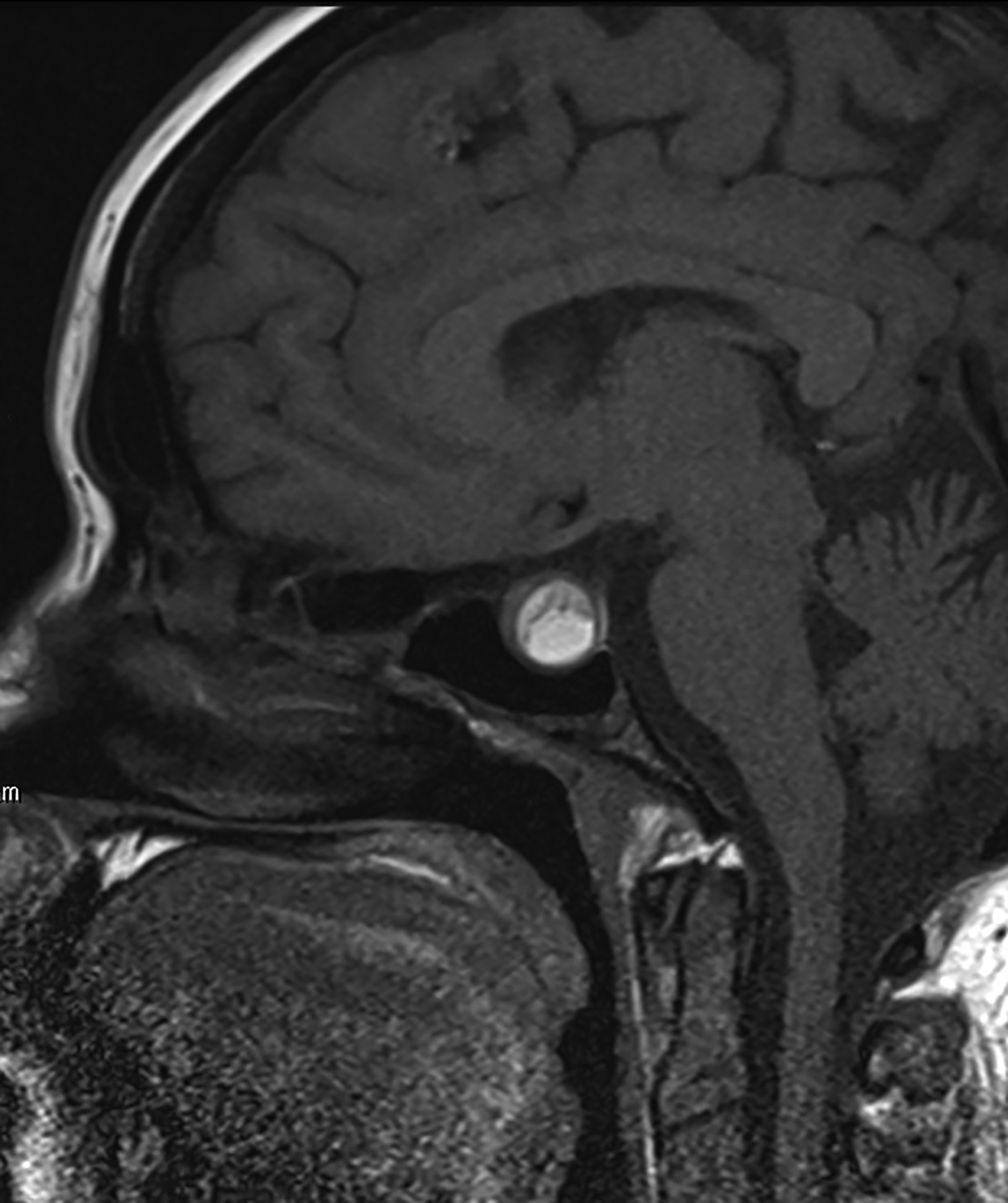
Rathke cleft cysts are non-neoplastic lesions that originate from remnants of the Rathke pouch. Owing to their epithelial origin, they are lined by columnar or cuboidal epithelium.16 They typically contain gelatinous fluid but can also contain hemorrhagic, mucinous, or inflammatory contents.17 Rathke cleft cysts are the most common lesions found incidentally in the sella and parasellar region, with an estimated incidence up to 11% postmortem.16
Most Rathke cleft cysts are usually asymptomatic. When they are symptomatic, headache is the most common presenting symptom, but others may include visual disturbances resulting from compression of the optic chiasm. Hormonal disturbances may occur as a result of pituitary and hypothalamic compression.18 Hormonal abnormalities may include hyperprolactinemia, cortisol deficiency, diabetes insipidus, syndrome of inappropriate antidiuretic hormone secretion, and hypogonadism.17
On CT, the density of Rathke cleft cysts varies according to their contents. Cysts may appear hyperdense, isodense, or hypodense.19 These noncalcified lesions have well-defined margins and typically do not show contrast enhancement.20 Their typical appearance on MRI reflects high proteinaceous content with T1 hyperintensity and T2 hypointensity (Figure 7).18 However, the signal intensity varies with the nature of the fluid contents.19,20 Intracystic hemorrhage may result in hyperintense signal on both T1 and T2 imaging. On postcontrast MRI, there should be no nodular enhancement, a feature that may differentiate these benign cysts from craniopharyngioma. Rarely, curvilinear enhancement is seen in the wall of the cyst.19
Conclusion
Intracranial cysts are seen in a broad spectrum of pathology with overlapping anatomic and imaging characteristics. However, proper knowledge of their clinical presentation, anatomic location, and imaging appearances, particularly on MRI, can help narrow the differential diagnosis and facilitate patient care.
References
- Bilginer B, Narin F, Hanalioglu S, Oguz KK, Akalan N. Virchow-Robin spaces cyst. Childs Nerv Syst. 2013;29(12):2157-2162.
- Gordon M, Parmar H, Ibrahim M. Spread of infection to Virchow-Robin spaces in a patient with Streptococcus pneumoniae meningitis. J Comput Assist Tomogr. 2009;33(4):562-564.
- Uchino A, Takase Y, Nomiyama K, Egashira R, Kudo S. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. 2006;16(4):905-914.
- Patibandla MR, Yerramneni VK, Mudumba VS, Manisha N, Addagada GC. Brainstem epidermoid cyst: An update. Asian J Neurosurg. 2016;11(3):194-200.
- Chowdhury FH, Haque MR, Sarker MH. Intracranial epidermoid tumor; microneurosurgical management: An experience of 23 cases. Asian J Neurosurg. 2013;8(1):21-28.
- Kato K, Ujiie H, Higa T, et al. Clinical presentation of intracranial epidermoids: a surgical series of 20 initial and four recurred cases. Asian J Neurosurg. 2010;5(1):32-40.
- Caldarelli M, Colosimo C, Di Rocco C. Intra-axial dermoid/epidermoid tumors of the brainstem in children. Surg Neurol. 2001;56(2):97-105.
- Lawrence JE, Nadarajah R, Treger TD, Agius M. Neuropsychiatric manifestations of colloid cysts: a review of the literature. Psychiatr Danub. 2015;27 Suppl 1:S315-320.
- pears RC. Colloid cyst headache. Curr Pain Headache Rep. 2004;8(4):297-300.
- Musa G, Simfukwe K, Gots A, Chmutin G, Chmutin E, Chaurasia B. Clinical and radiological characteristics in fatal third ventricle colloid cyst. Literature review. J Clin Neurosci. 2020;82(Pt A):52-55.
- Jenkinson MD, Mills S, Mallucci CL, Santarius T. Management of pineal and colloid cysts. Pract Neurol. 26 2021;21(4):292-299.
- Mustansir F, Bashir S, Darbar A. Management of arachnoid cysts: a comprehensive review. Cureus. 10 2018;10(4):e2458.
- Al-Holou WN, Terman S, Kilburg C, Garton HJ, Muraszko KM, Maher CO. Prevalence and natural history of arachnoid cysts in adults. J Neurosurg. 2013;118(2):222-231.
- Karnazes AC, Kei J, Le MV. Image diagnosis: arachnoid cyst. Perm J. 2015;19(2):e110-111.
- Dutt SN, Mirza S, Chavda SV, Irving RM. Radiologic differentiation of intracranial epidermoids from arachnoid cysts. Otol Neurotol. 2002;23(1):84-92.
- Larkin S, Karavitaki N, Ansorge O. Rathke’s cleft cyst. Handb Clin Neurol. 2014;124:255-269.
- Cabuk B, Selek A, Emengen A, Anik I, Canturk Z, Ceylan S. Clinicopathologic characteristics and endoscopic surgical outcomes of symptomatic Rathke’s cleft cysts. World Neurosurg. 2019;132:e208-e216.
- Crenshaw WB, Chew FS. Rathke’s cleft cyst. AJR Am J Roentgenol. Jun 1992;158(6):1312.
- Nishioka H, Haraoka J, Izawa H, Ikeda Y. Magnetic resonance imaging, clinical manifestations, and management of Rathke’s cleft cyst. Clin Endocrinol (Oxf). 2006;64(2):184-188.
- Kucharczyk W, Peck WW, Kelly WM, Norman D, Newton TH. Rathke cleft cysts: CT, MR imaging, and pathologic features. Radiology.1987;165(2):491-495.
Citation
O K, H K, SS K, I Z.Non-neoplastic Cystic Lesions of the Central Nervous System, Part 1: Developmental Cysts. Appl Radiol. 2022; (4):21-26.
July 14, 2022