Neuroimaging of pediatric abusive head trauma
Images


Abusive head trauma (AHT) is a major cause of morbidity and mortality in children subjected to abuse, accounting for nearly one-third of all deaths caused by child abuse.1 Those children who do survive AHT are often left with significant and permanent disabilities, including motor and visual deficits, language abnormalities, seizures, and behavioral problems.2-4 Despite the severe consequences of AHT, no standard criteria or objective tests exist for differentiating AHT from accidental trauma.5 Clinical histories and presentations are often unclear and contribute to unrecognized cases or delays in diagnosis. Recent studies suggest healthcare providers had previously seen nearly one-third of children who subsequently died from AHT in the time leading up to their death.6,7 In some cases, delay in diagnosis has been attributed to misinterpretation of radiologic studies, highlighting the need for improved education and awareness of the appropriate imaging techniques in the evaluation of AHT as well as common radiologic findings.6-10
Efforts continue to be made to understand the underlying pathogenesis and mechanisms of AHT. The two major categories of AHT include shaking mechanisms, in which repetitive acceleration-deceleration forces typically result in subdural hematomas (SDH), retinal hemorrhages and global parenchymal damage; and direct impact trauma, which may result in skull fractures and focal coup/contrecoup parenchymal injuries. However, the exact mechanisms of injury are often unknown and may result from a combination of forces. Parenchymal damage further can be multifactorial; for example, intracranial hemorrhage, hypoxic-ischemic injury and axonal disruption may all result in cytotoxic edema.11-13
Noncontrast head computerized tomography (CT) followed by conventional magnetic resonance imaging (MRI) is widely considered to be the first step in evaluating suspected AHT, with diffusion-weighted imaging (DWI) and susceptibility-weighted imaging (SWI) being critical MR sequences.14-20 Diffusion tensor imaging (DTI), magnetic resonance spectroscopy (MRS) and arterial spin labeling (ASL) perfusion imaging of the brain also may be utilized, though these sequences are not typically included in routine protocols. In addition to evaluating brain injury, increasing emphasis has been placed on imaging of the orbits, olfactory tracts, and cervical spine. We outline current imaging techniques appropriate for the evaluation of AHT, highlighting their unique contribution to obtaining an accurate and timely diagnosis, as well as common radiologic findings.
Imaging modalities
Computerized tomography
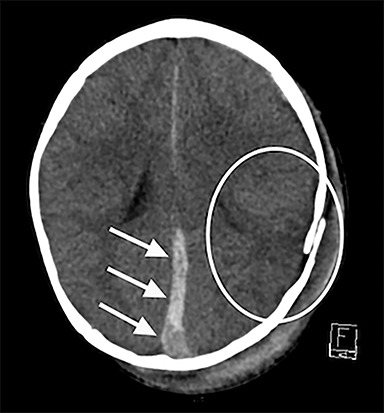
Noncontrast head CT is considered the appropriate primary study for children who present with signs and symptoms of head trauma including loss of consciousness, confusion, vomiting, irritability, seizures, or respiratory difficulties. CT is rapid, cost effective, widely available, and able to reveal injuries that may need rapid surgical intervention, including hemorrhage, midline shift, and herniation. Contrast should generally be avoided, as it can obscure high-density acute hemorrhage often found in AHT. Although the risk of ionizing radiation associated with CT should always be a consideration, it is widely accepted that the benefits of obtaining a CT in substantial trauma far outweigh the risks. Furthermore, in cases of suspected trauma when clinical evaluation may be difficult or indeterminate, or in cases when abuse is strongly suspected even without major neurological symptoms, head CT should be obtained, as studies have shown CT can detect occult head trauma even in cases of children with normal exams.14,21 While older studies suggest the use of facial or skull radiographs to detect fractures associated with AHT, recent evidence suggests CT is superior to skull or facial radiographs for detecting fractures, and should be obtained with three-dimensional reconstructions to distinguish fractures from enlarged sutures in children less than two years of age15,16 (Figure 1A-B). Thus, facial or skull radiographs are often not necessary and may only serve as a source of supplemental information.
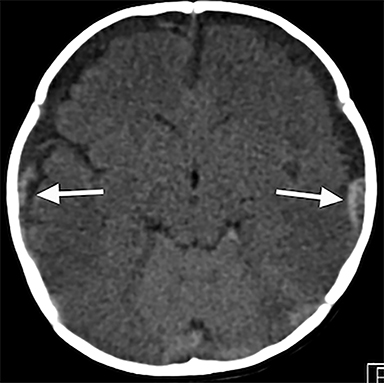
Noncontrast CT can reliably identify substantial SDH (Figure 1A). This is critical, as SDH are significantly associated with AHT,17,18 with estimates suggesting that SDH may be present in up to 90% of children who experience AHT.19 However, SDH can also be a consequence of the birthing process,20,22 benign enlargement of the subarachnoid spaces,23 intracranial congenital malformations,24 coagulation and hematologic disorders,25 and certain metabolic disorders, most commonly glutaric aciduria.26 Therefore, in addition to clinical history, SDH location and pattern is important. In particular, interhemispheric, convexity, and posterior fossa hematomas are individually associated with AHT17 and significantly elevated in AHT versus accidental trauma27 (Figure 1A). Mixed-density SDH are also more frequent in AHT (67% vs 18%), while homogenous hyperdense SDH were more frequent in accidental trauma (74% vs 33%).28 It is important to note that spontaneous rebleeding into chronic SDH in infants is less likely than adults, and thus some suggest that mixed SDH in infants should raise further suspicions for repetitive head injury.28 As blood attenuation is often multifactorial, especially in the acute setting, follow-up CT or MRI exams are usually necessary in order to reliably determine the age of a hematoma.28
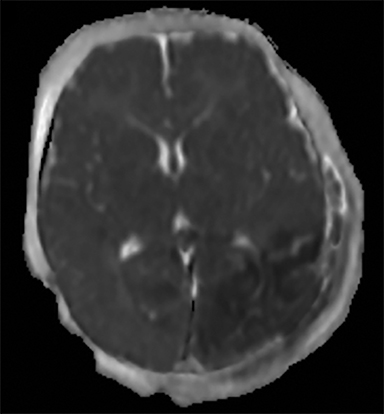
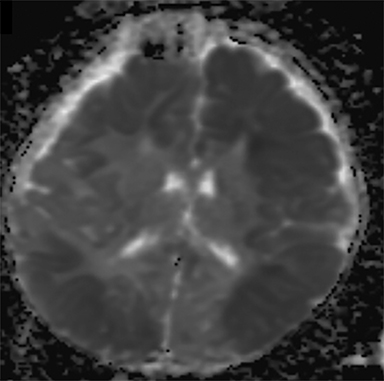
Noncontrast CT is also useful for detecting the reversal sign, a phenomenon in which there is an inversion of the normal attenuation relationship between gray and white matter where gray matter has lower attenuation than white matter and there is an increased attenuation of the thalami, brainstem, and cerebellum.29 The reversal sign is often a marker of diffuse cerebral edema, a common finding in AHT (Figure 2A). Positive cases of acute trauma on CT should be evaluated in conjunction with head MRI within 24-48 hours to better define extent of injury. Additionally, if the head CT is negative but trauma is strongly suspected by clinical evaluation, MRI should be obtained, as CT is often negative in the acute setting.
Magnetic resonance imaging
Conventional —Conventional MRI including gradient echo (GRE), T1-weighted, T2-weighted, and fluid-attenuated inversion recovery (FLAIR) is routinely performed in suspected cases of AHT in order to better characterize intraparenchymal injuries.24 While MRI is more technically challenging, time-consuming and often requires anesthesia, the additional insight obtained from a head MRI is crucial for both initial and follow-up imaging for AHT. Conventional sequences in particular are better able to demonstrate recurrent episodes and evolution of injury by helping to date subacute and chronic hematomas, combating one of the challenges of CT (Figure 1C). T1-weighted imaging will often demonstrate hyperintensity of the cortical ribbon, identifying hemorrhagic cortical contusions or laminar necrosis. Identifying ischemia on T2-weighted imaging is challenging in infants and young children due to the high water content of unmyelinated white matter; however, loss of gray-white distinction along cortex and in deep nuclei can be a clue.
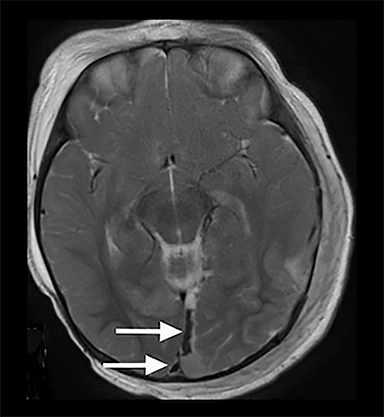
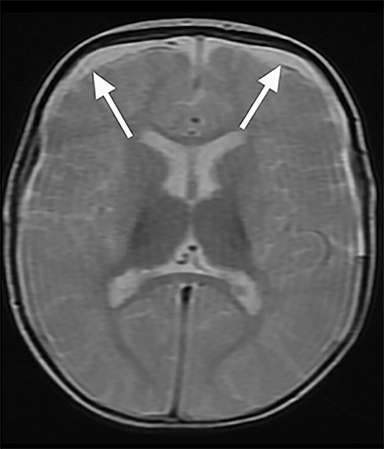
Conventional MRI sequences have also been shown to detect additional injuries not initially detected by CT including SDH, shearing injuries, ischemia, and parenchymal hemorrhages.30,31 One study reported new findings in 25% (95% CI: 18.3 – 33.16%) of children when an MRI was conducted after an abnormal early CT.30 An example of such a finding not well seen on CT is parenchymal lacerations; a retrospective review of 165 cases of pediatric accidental and abusive head trauma found that only half of the lacerations detected on MRI were appreciable on CT.31 Parenchymal lacerations in this study were only seen in AHT cases.31 Isolated subdural hygromas and those associated with subacute hematomas are also better detected by MRI and can be associated with AHT32 (Figure 3A-B). In order to avoid the use of anesthesia, as well as potentially eliminate the need for an initial CT, the use of rapid MRI sequences has gained recent attention in evaluation of intracranial pathology in the pediatric population; however, a recent study suggests ultrafast MRI, even in combination with noncontract CT, has lower sensitivity compared to conventional MRI in patients with suspected AHT33 and therefore is not generally used in this setting.
Diffusion-weighted imaging—In addition to conventional MRI series, DWI and the apparent diffusion coefficient (ADC) map have a well-established role in evaluating AHT, being crucial in evaluation of parenchymal injury.34-36 Broadly, DWI and ADC mapping have been shown to be very sensitive in detecting early nonhemorrhagic infarction and subsequent acute cytotoxic edema, often hours to days before changes on CT.30,37-39 These types of injuries are common in AHT and often result in poor outcomes including seizures, intubation, neurosurgical procedures, and inpatient rehabilitation.34,40,41 DWI can help recognize these injuries in a timely manner, and can also be particularly helpful in children under two years of age in whom it is difficult to distinguish ischemic injury from normal immaturely myelinated white matter.
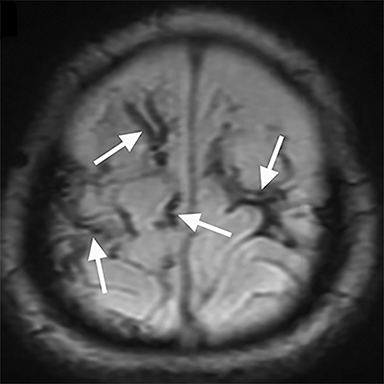
DWI often reveals more extensive injury than conventional sequences (Figure 1D).38,39,42 Widespread areas of diffusion restriction are common in AHT (Figure 2B, 3C). Diffuse supratentorial or posterior cerebral are the most frequently reported patterns (Figure 4A). Arterial watershed hypoxic ischemia, venous hemorrhage/infarction, diffuse axonal injury (DAI), and contusions are less commonly encountered.43 Although the shaking mechanism predisposes to DAI, incidences are overall less common in AHT compared to accidental trauma.18,27 Low ADC values have also been found in several studies to be associated with poor long-term neurodevelopmental outcomes, offering additional prognostic value.44-48 Importantly, DWI can also identity new areas of injury on repeat scans weeks after initial injury.
DTI, a form of DWI, is another imaging modality with growing applications. DTI is currently considered a useful technique in the evaluation of microstructural white matter abnormalities, as it is a direct measure of injury to axonal fibers.49 While DTI has most commonly been used to assess myelination during maturation,50 new investigations have focused on application of DTI to head trauma in children, demonstrating correlation of changes on DTI with injury severity, functional outcome, and long-term recovery.49,51 One study directly assessing DTI in cases of AHT demonstrated unique widespread reduced axial diffusivity (AD) in white matter regions in AHT compared to controls, with localization to white matter tracts that meditate neurocognitive domains including auditory, visual, and executive functions. These findings suggest that DTI is able to detect additional white matter changes that may be helpful in predicting long-term outcomes; however, it is also important to note that a negative DTI does not exclude AHT.
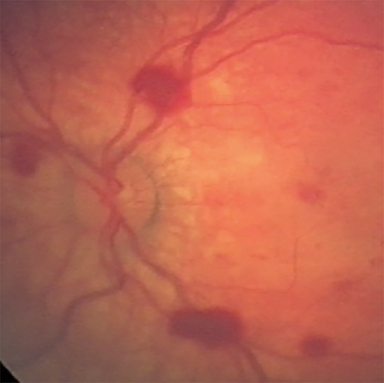
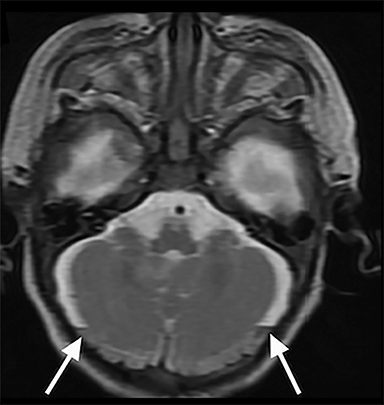
Susceptibility-weighted imaging—SWI is a 3D high-resolution MRI technique that accentuates the paramagnetic properties of blood products. SWI is more sensitive than conventional MRI, including GRE, in detecting hemorrhagic lesions. Examples of better visualized abnormalities include hemorrhagic foci associated with DAI,52,53 clot formation within injured bridging veins, and hemosiderin deposition in superficial siderosis32,54,55 (Figure 3D). Bridging vein thrombosis, in particular, is considered a sign of traumatic SDH in the context of AHT, as evidenced by blooming within bridging veins on SWI and often without signs of venous infarction.56,57 In both accidental and AHT, the extent and number of hemorrhages is strongly correlated with initial severity of injury and long-term severity of neurologic outcome months after injury, as well as neuropsychological functioning several years after injury in AHT patients.53,58,59 SWI is also increasingly utilized in conjunction with the funduscopic exam to identify retinal hemorrhages, a highly specific finding of AHT56,57,60 (Figure 2C-D).
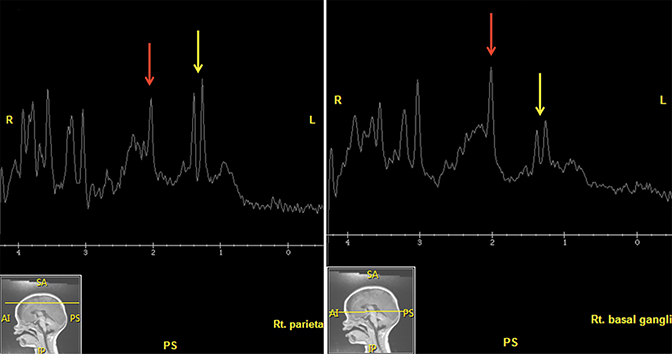
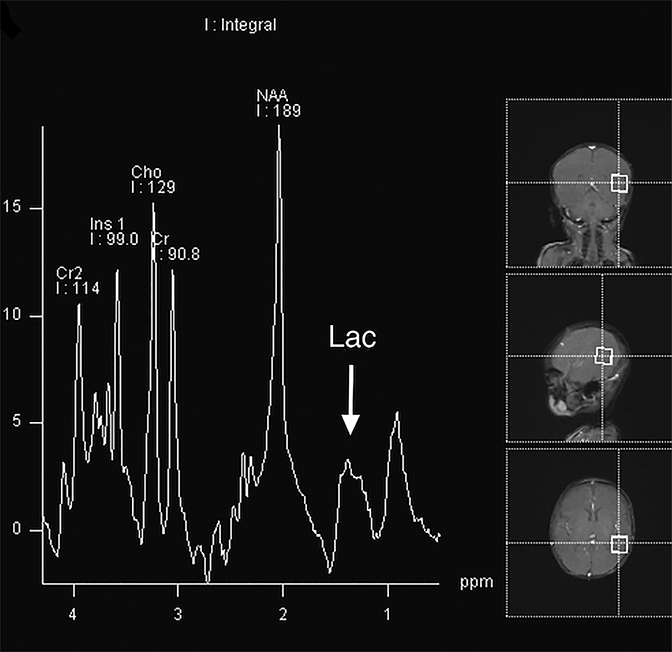
MR Spectroscopy—MRS is a noninvasive imaging technique that acquires concentration levels of various metabolites that are indicative of underlying neuronal dysfunction.61 In processes that destroy brain tissue, N-acetyl aspartate (NAA), a neuronal marker, is reduced; likewise, choline, a cell membrane marker, is increased. Lactate elevation is widely known to result from hypoxic-ischemic damage as neurons switch to anaerobic metabolism.62 While MRS is not routinely used in the acute trauma setting, it can help confirm the extent of neuronal injury in severe cases such as those resulting in hypoxic-ischemic injury and DAI. Several studies have shown that decreased NAA or NAA/creatine (Cr) ratios in children with AHT or TBI from other causes portend a worse outcome;58,63-66 two of these studies found similar prognoses in patients with elevated lactate64,66 (Figures 3E, 5A). Additional studies found increased rates of glutamate/glutamine67 and myoinositol68 to be associated with poor prognosis in children with TBI. Confirming diffuse brain injury with spectroscopy, if available, can aid in early management and intervention.
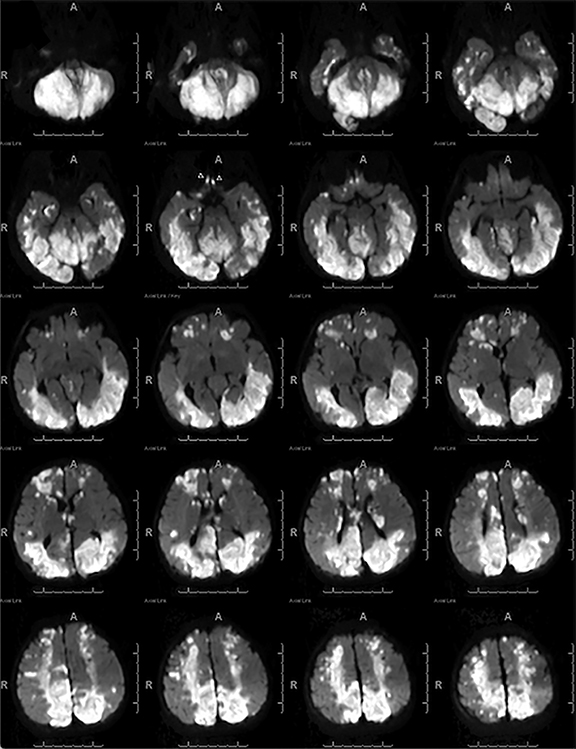
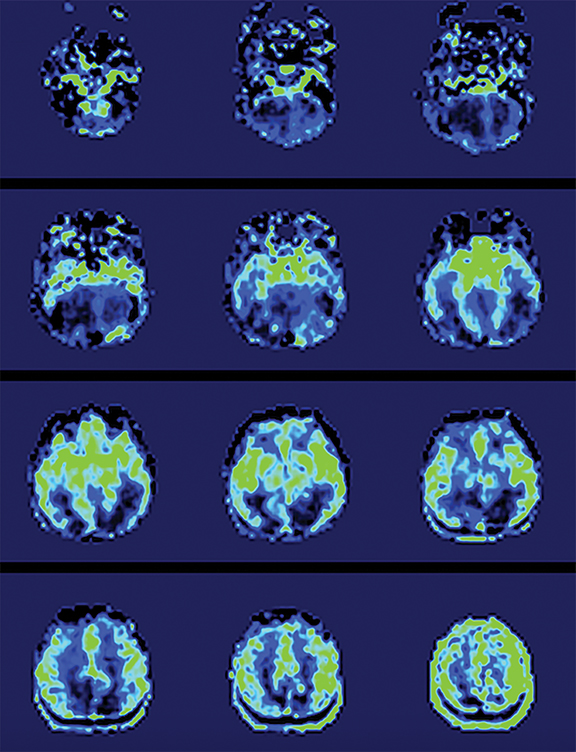
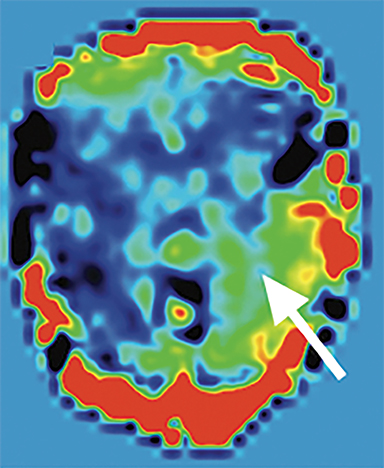
Arterial spin labeling—As one of the two most common MRI perfusion-imaging methods, along with dynamic susceptibility contrast (DSC), ASL can also be utilized to evaluate TBI. Instead of contrast, ASL uses magnetically labeled water molecules in arterial blood as an endogenous tracer, making ASL a noninvasive technique for quantifying cerebral blood flow. Studies on ASL in mild accidental brain trauma have described diminished cerebral blood flow in certain brain regions in acute/subacute stages which was later restored in the chronic stage69 and have shown that hypoperfusion may be seen in mild TBI in the context of an otherwise negative MRI, both acutely and months after injury.69,70 In chronic TBI, encephalomalacia is the main contributor to alternation in absolute cerebral blood flow.71 ASL may also detect additional injury in cases of AHT which are not as evident on other sequences (Figure 5B-C) or may help to confirm areas of restricted diffusion on DWI (Figure 4B). An investigation of perfusion using ASL in patients with AHT found children with AHT had more perfusion abnormalities compared to those without AHT and children with the most significant hypoperfusion had the poorest clinical outcomes.72 Further studies using ASL should be pursued in order to solidify its role in initial and subsequent evaluation of AHT.
Other imaging considerations
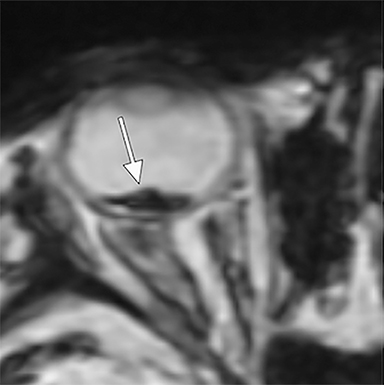
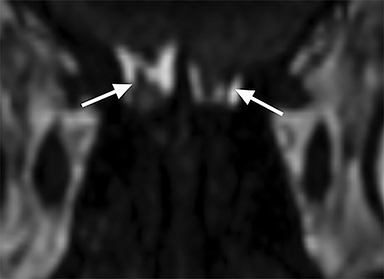
When AHT is suspected, special attention should be paid to the retina and other orbital structures. As noted above, retinal hemorrhages are considered highly specific for AHT. They are often bilateral, extensive and multilayered in this context, and their presence indicates an acceleration-deceleration type mechanism. Intravitreous and optic nerve sheath hemorrhage and/or optic atrophy are other ophthalmologic findings of AHT that may be visible on imaging. In addition, trauma to the cisternal olfactory tracts and bulbs are frequently encountered as the result of severe coup/contrecoup injury or shearing across the cribriform plate,73,74 which can be sustained from either a direct head strike or shaking (Figure 6). High-resolution imaging of the orbits, olfactory bulbs, and other cisternal structures can be readily performed with balanced coherent steady-state free precession (bSSFP) imaging. This sequence provides superior contrast for fine structures that are profiled by fluid or fat, making it ideal for delineating the small anatomic structures of the orbits, skull base, and exiting craniospinal nerves.
Finally, as small children typically lack the neck strength and head control to protect themselves against sudden hyper-extension/flexion forces, they are more prone to cervical spine injury, including spinal subdural hematomas and ligamentous injury.75-79 Increasing evidence suggests cervical spine injury is more common AHT and is associated with more severe brain injury and poor outcomes; thus, cervical spine MRI is also now generally warranted.75,77,79-81
Conclusion
The evaluation of pediatric AHT is an ongoing diagnostic challenge as there are currently no standard criteria for differentiating AHT from accidental trauma. Clinical presentation and histories are often nonspecific, and thus investigations always warrant a thorough, multi-disciplinary approach including skeletal surveys and ophthalmologic exam. Neuroradiologic imaging is vital in the evaluation of suspected cases, potentially offering insight into the mechanisms of injury and long-term prognosis. With the use of more advanced imaging such as DWI, DTI, SWI and MRS, prediction of long-term outcomes and identification of those at high risk is becoming more feasible, offering an opportunity to intervene earlier.
References
- Palusci VJ, Covington TM. Child maltreatment deaths in the U.S. National Child Death Review Case Reporting System. Child Abuse Negl. 2014;38(1):25-36.
- Lind K, Toure H, Brugel D, et al. Extended follow-up of neurological, cognitive, behavioral and academic outcomes after severe abusive head trauma. Child Abuse Negl. 2016;51:358-367.
- Barlow KM, Thomson E, Johnson D, et al. Late neurologic and cognitive sequelae of inflicted traumatic brain injury in infancy. Pediatrics. 2005;116(2):e174-185.
- Keenan HT, Runyan DK, Marshall SW, et al. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114(3):633-639.
- Estroff JM, Foglia RP, Fuchs JR. A comparison of accidental and nonaccidental trauma: It is worse than you think. J Emerg Med. 2015;48(3):274-9.
- Jenny C, Hymel KP, Ritzen A, et al. Analysis of missed cases of abusive head trauma. JAMA. 1999;281(7):621-626.
- King WK, Kiesel EL, Simon HK. Child abuse fatalities: Are we missing opportunities for intervention? Pediatr Emerg Care. 2006;22(4):211-214.
- Letson MM, Cooper JN, Deans KJ, et al. Prior opportunities to identify abuse in children with abusive head trauma. Child Abuse Negl. 2016; 60:36-45.
- Ortega HW, Vander Velden H, Kreykes NS, et al. Childhood death attributable to trauma: is there a difference between accidental and abusive fatal injuries? J Emerg Med. 2013;45(3):332-337.
- Kralik SF, Finke W, Wu IC, et al. Radiologic head CT interpretation errors in pediatric abusive and non-abusive head trauma patients. Pediatr Radiol. 2017;47(8):942-951.
- Geddes JF, Hackshaw AK, Vowles GH, et al. Neuropathology of inflicted head injury in children. Patterns of brain damage. Brain. 2001;124(Pt 7):1290-1298.
- Geddes JF, Vowles GH, Hackshaw AK, et al. Neuropathology of inflicted head injury in children. II. Microscopic brain injury in infants. Brain. 2001;124(Pt 7):1299-1306.
- Nixon JN, Soares BP. Imaging of abusive head trauma: A review and update. Curr Radiol Rep. 2016;4(8).
- Lasky AL, Hosti M, Runyan DK, et al. Occult head trauma in young suspected victims of physical abuse. J Pediatr. 2004;144(6):719-722.
- Ryan ME, Palasis S, Saigal G, et al. ACR appropriateness criteria head trauma—child. J Am Coll Radiol. 2014;11(10):939-947.
- Culotta PA, Crowe JE, Tran QA, et al. Performance of computed tomography of the head to evaluate for skull fractures in infants with suspected non-accidental trauma. Pediatr Radiol. 2017;47(1):74-81.
- Kemp AM, Jaspan T, Griffiths J, et al. Neuroimaging: what neuroradiological features distinguish abusive from non-abusive head trauma? A systematic review. Arch Dis Child. 2011;96(12):1103-12.
- Piteau SJ, Ward MG, Barrowman NJ, et al. Clinical and radiographic characteristics associated with abusive and nonabusive head trauma: a systematic review. Pediatrics. 2012; 130(2):315-323.
- Kelly P, John S, Vincent AL, et al. Abusive head trauma and accidental head injury: a 20- year comparative study of referrals to a hospital child protection team. Arch Dis Child. 2015;100(12):1123-1130.
- Whitby EH, Griffiths PD, Rutter S, et al. Frequency and natural history of subdural haemorrhages in babies and relation to obstetric factors. Lancet. 2004;363(9412):846-851.
- Rubin DM, Christian CW, Bilaniuk LT, et al. Occult head injury in high-risk abused children. Pediatrics. 2003;111(6 Pt 1):1382-1386.
- Rooks VJ, Eaton JP, Ruess L, et al. Prevalence and evolution of intracranial hemorrhage in asymptomatic term infants. AJNR Am J Neuroradiol. 2008;29(6):1082-9.
- McKeag H, Christian CW, Rubin D, et al. Subdural hemorrhage in pediatric patients with enlargement of the subarachnoid spaces. J Neurosurg Pediatr. 2013;11(4):438-444.
- Girard N, Brunel H, Dory-Lautrec P, et al. Neuroimaging differential diagnoses to abusive head trauma. Pediatr Radiol. 2016;46(5):603-614.
- Jackson J, Carpenter S, Anderst J. Challenges in the evaluation for possible abuse: presentations of congenital bleeding disorders in childhood. Child Abuse Negl. 2012;36(2):127-134.
- Morris AA, Hoffmann GF, Naughten ER, et al. Glutaric aciduria and suspected child abuse. Arch Dis Child. 1999;80(5):404-405.
- Ewing-Cobbs L, Prasad M, Kramer L, et al. Acute neuroradiologic findings in young children with inflicted or noninflicted traumatic brain injury. Childs Nerv Syst. 2000;16(1):25-33.
- Tung GA, Kumar M, Richardson RC, et al. Comparison of accidental and nonaccidental traumatic head injury in children on noncontrast computed tomography. Pediatrics. 2006;118(2):626-633.
- Kavanagh EC. The reversal sign. Radiology. 2007;245(3):914-915.
- Kemp AM, Rajaram S, Mann M, et al. What neuroimaging should be performed in children in whom inflicted brain injury (iBI) is suspected? A systematic review. Clin Radiol. 2009;64(5):473-483.
- Palifka LA, Frasier LD, Metzger RR, et al. Parenchymal brain laceration as a predictor of abusive head trauma. AJNR Am J Neuroradiol. 2016;37(1):163-168.
- Hahnemann ML, Kinner S, Schweiger B, et al. Imaging of bridging vein thrombosis in infants with abusive head trauma: the “Tadpole Sign”. Eur Radiol. 2015;25(2):299-305.
- Kralik SF, Yasrebi M, Supakul N, et al. Diagnostic performance of ultrafast brain MRI for evaluation of abusive head trauma. AJNR Am J Neuroradiol. 2017;38(4):807-813.
- Kemp AM, Butler A, Morris S, et al. Which radiological investigations should be performed to identify fractures in suspected child abuse? Clin Radiol. 2006;61(9):723-736.
- Meyer JS, Gunderman R, Coley BD, et al. ACR Appropriateness Criteria((R)) on suspected physical abuse-child. J Am Coll Radiol. 2011;8(2):87-94.
- Kleinman P, Di Pietro M, Brody A, et al. Diagnostic imaging of child abuse. Pediatrics. 2009; 123(5):1430-1435.
- Ashwal S, Wycliffe ND, Holshouser BA. Advanced neuroimaging in children with nonaccidental trauma. Dev Neurosci. 2010;32(5-6):343-60.
- Suh DY, Davis PC, Hopkins KL, et al. Nonaccidental pediatric head injury: diffusionweighted imaging findings. Neurosurgery. 2001;49(2):309-318; discussion 18-20.
- Biousse V, Suh DY, Newman NJ, et al. Diffusion-weighted magnetic resonance imaging in Shaken Baby Syndrome. Am J Ophthalmol. 2002;133(2):249-255.
- Ichord RN, Naim M, Pollock AN, et al. Hypoxic-ischemic injury complicates inflicted and accidental traumatic brain injury in young children: the role of diffusion-weighted imaging. J Neurotrauma. 2007;24(1):106-118.
- Tun K, Choudhary AK, Methratta S, et al. radiological features of nonaccidental injury. J Radiol Nurs. 2013;32(1):3-9.
- Parizel PM, Ceulemans B, Laridon A, et al. Cortical hypoxic-ischemic brain damage in shaken-baby (shaken impact) syndrome: value of diffusion-weighted MRI. Pediatr Radiol. 2003;33(12):868-871.
- Zimmerman RA, Bilaniuk LT, Farina L. Non-accidental brain trauma in infants: diffusion imaging, contributions to understanding the injury process. J Neuroradiol. 2007;34(2):109-14.
- Tanoue K, Aida N, Matsui K. Apparent diffusion coefficient values predict outcomes of abusive head trauma. Acta paediatr. 2013;102(8):805-808.
- Babikian T, Tong KA, Galloway NR, et al. Diffusion-weighted imaging predicts cognition in pediatric brain injury. Pediatr Neurol. 2009;41(6):406-412.
- Galloway NR, Tong KA, Ashwal S, et al. Diffusion-weighted imaging improves outcome prediction in pediatric traumatic brain injury. J Neurotrauma. 2008;25(10):1153-1162.
- Hergan K, Schaefer PW, Sorensen AG, et al. Diffusion-weighted MRI in diffuse axonal injury of the brain. Eur Radiol. 2002;12(10):2536-2541.
- Skandsen T, Finnanger TG, Andersson S, et al. Cognitive impairment 3 months after moderate and severe traumatic brain injury: a prospective follow-up study. Arch Phys Med Rehabil. 2010;91(12):1904-1913.
- Hunter JV, Wilde EA, Tong KA, et al. Emerging imaging tools for use with traumatic brain injury research. J Neurotrauma. 2012;29(4):654-671.
- McGraw P, Liang L, Provenzale JM. Evaluation of normal age-related changes in anisotropy during infancy and childhood as shown by diffusion tensor imaging. AJR Am J Roentgenol. 2002;179:1515-1522.
- Wozniak JR, Krach L, Ward E, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsychol. 2007;22(5):555-568.
- Ashwal S, Babikian T, Gardner-Nichols J, et al. Susceptibility-weighted imaging and proton magnetic resonance spectroscopy in assessment of outcome after pediatric traumatic brain injury. Arch Phys Med Rehabil. 2006;87(12 Suppl 2):S50-58.
- Tong KA, Ashwal S, Holshouser BA, et al. Hemorrhagic shearing lesions in children and adolescents with posttraumatic diffuse axonal injury: improved detection and initial results. Radiology. 2003;227(2):332-9.
- Adamsbaum C, Rambaud C. Abusive head trauma: don’t overlook bridging vein thrombosis. Pediatr Radiol. 2012;42(11):1298-1300.
- Yilmaz U, Korner H, Meyer S, et al. Multifocal signal loss at bridging veins on susceptibility-weighted imaging in abusive head trauma. Clin Neuroradiol. 2015;25(2):181-185.
- Hwang M, Shin SS, Thimm MA, et al. Apparent diffusion coefficient (ADC) of the vitreous humor and susceptibility weighted imaging (SWI) of the retina in abused children with retinal hemorrhages. Clin Imaging. 2017;44:38-41.
- Zuccoli G, Panigrahy A, Haldipur A, et al. Susceptibility weighted imaging depicts retinal hemorrhages in abusive head trauma. Neuroradiology. 2013;55(7):889-893.
- Tong KA, Ashwal S, Holshouser BA, et al. Diffuse axonal injury in children: clinical correlation with hemorrhagic lesions. Ann Neurol. 2004;56(1):36-50.
- Colbert CA, Holshouser BA, Aaen GS, et al. Value of cerebral microhemorrhages detected with susceptibility-weighted MR imaging for prediction of long-term outcome in children with nonaccidental trauma. Radiology. 2010;256(3):898-905.
- Gencturk M, Tore H, Nascene D, et al. Various cranial and orbital imaging findings in pediatric abusive and non-abusive head trauma, and relation to outcomes. Clin Neuroradiol. 2018;1-9.
- Ashwal S, Holshouser BA, Tong KA. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Dev Neurosci. 2006;28(4-5):309-326.
- Cecil KM, Jones BV. Magnetic resonance spectroscopy of the pediatric brain. Top Magn Reson Imaging. 2001;12(6):435-452.
- Hunter JV, Thornton RJ, Wang ZJ, et al. Late proton MR spectroscopy in children after traumatic brain injury: correlation with cognitive outcomes. AJNR Am J Neuroradiol. 2005;26(3):482-488.
- Ashwal S, Holshouser BA, Shu SK, et al. Predictive value of proton magnetic resonance spectroscopy in pediatric closed head injury. Pediatr Neurol. 2000;23(2):114-125.
- Haseler LJ, Arcinue E, Danielsen ER, et al. Evidence from proton magnetic resonance spectroscopy for a metabolic cascade of neuronal damage in shaken baby syndrome. Pediatrics. 1997;99(1):4-14.
- Makoroff KL, Cecil KM, Care M, et al. Elevated lactate as an early marker of brain injury in inflicted traumatic brain injury. Pediatr Radiol. 2005;35(7):668-76.
- Ashwal S, Holshouser B, Tong K, et al. Proton MR spectroscopy detected glutamate/glutamine is increased in children with traumatic brain injury. J Neurotrauma. 2004;21:1539-52.
- Ashwal S, Holshouser B, Tong K, et al. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr Res. 2004;56(4):630-638.
- Peng SP, Li YN, Liu J, et al. Pulsed arterial spin labeling effectively and dynamically observes changes in cerebral blood flow after mild traumatic brain injury. Neural Regen Res. 2016;11(2):257-261.
- Lin CM, Tseng YC, Hsu HL, et al. Arterial spin labeling perfusion study in the patients with subacute mild traumatic brain injury. PloS One. 2016;11(2):e0149109.
- Kim J, Whyte J, Patel S, et al. Resting cerebral blood flow alterations in chronic traumatic brain injury: an arterial spin labeling perfusion FMRI study. J Neurotrauma. 2010;27(8):1399- 1411.
- Wong A, Yeh C, Liu H, et al. Arterial spin-labeling perfusion imaging of children with subdural hemorrhage: Perfusion abnormalities in abusive head trauma. J Neuroradiol. 2017;44(4):281-287.
- Sandford AA, Davidson TM, Herrera N, et al. Olfactory dysfunction: a sequela of pediatric blunt head trauma. Int J Pediatr Otorhinolaryngol. 2006;70(6):1015-1025.
- Doty R, Yousem D, Pham L, et al. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997;54:1131-1140.
- Choudhary AK, Ishak R, Zacharia TT, et al. Imaging of spinal injury in abusive head trauma: a retrospective study. Pediatr Radiol. 2014; 44(9):1130-1140.
- Kadom N, Khademian Z, Vezina G, et al. Usefulness of MRI detection of cervical spine and brain injuries in the evaluation of abusive head trauma. Pediatr Radiol. 2014;44(7):839-848.
- Jacob R, Cox M, Koral K, et al. MR imaging of the cervical spine in nonaccidental trauma: A tertiary institution experience. AJNR Am J Neuroradiol. 2016;37(10):1944-1950.
- Koumellis P, McConachie NS, Jaspan T. Spinal subdural haematomas in children with non-accidental head injury. Arch Dis Child. 2009;94(3):216-219.
- Governale LS, Brink FW, Pluto CP, et al. A retrospective study of cervical spine MRI findings in children with abusive head trauma. Pediatr Neurosurg. 2018;53(1):36-42.
- Katz JS, Oluigbo CO, Wilkinson CC, et al. Prevalence of cervical spine injury in infants with head trauma. J Neurosurg Pediatr. 2010;5(5):470-473.
- Choudhary AK, Bradford R, Dias MS, et al. Spinal subdural hemorrhage in abusive head trauma: A retrospective study. Radiology. 2012;262(1):216-223.
Citation
L NDDK.Neuroimaging of pediatric abusive head trauma. Appl Radiol. 2019; (3):22-27.
June 7, 2019