MRI of the brachial plexus: A practical review
Images








Diagnosing brachial plexus pathology can be clinically challenging, often necessitating further evaluation with MRI. Owing to its vague symptomatology, uncommon nature, and complex anatomy, the brachial plexus presents a diagnostic dilemma to clinicians and radiologists alike and has been the subject of many prior reviews offering various perspectives on its imaging and pathology.1-5 The objective of this review is to provide the general radiologist with an up-to-date, practical approach to understanding the anatomy, pathology, and imaging of the brachial plexus.
Imaging anatomy of the brachial plexus
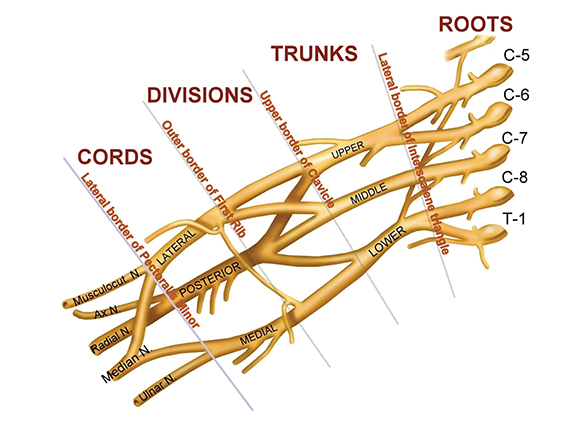
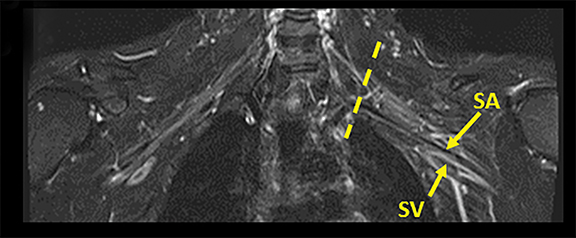
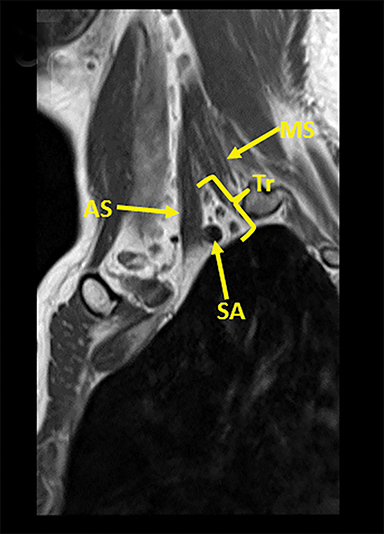
The brachial plexus (BP) provides sensory and motor innervation to the ipsilateral shoulder, chest, arm, and hand. Arising from the C5-T1 ventral rami of the spinal cord, the brachial plexus is divided anatomically into roots, trunks, divisions and cords (Figure 1). Upon exiting their respective neural foramina, the roots travel in the interscalene space, bounded anteriorly by the anterior scalene muscle, posteriorly by the middle/posterior scalene muscles, and inferiorly by the subclavian artery/first rib.2, 6 At the lateral aspect of the middle scalene muscle, the upper two roots (C5 & C6) join to form the upper trunk, the middle root (C7) continues on as the middle trunk, and the lower two roots (C8 & T1) join to form the lower trunk.1, 2, 6 The trunks course supero-posterior to the subclavian artery and each divides into anterior and posterior divisions in the costoclavicular triangle. The costoclavicular triangle has the following boundaries: the clavicle superiorly, subclavius muscle anteriorly, and the first rib and middle scalene muscles posteriorly.1,2,6 At the lateral border of the first rib, the divisions unite to form the medial, lateral, and posterior cords according to their relation to the ipsilateral subclavian/axillary artery.1,2,5
Terminal branches and innervation
The cords in turn divide into the terminal nerves: ulnar, median, musculocutaneous, radial, and axillary at the border of the pectoralis minor muscle.1, 2, 6 Smaller branches arise from various segments of the brachial plexus, and are depicted in the graphical representation of the brachial plexus (Figure 1). Various segments of the brachial plexus innervate several important structures. Specifically, the posterior cord innervates the latissimus dorsi, teres major, and subscapularis muscles. The supraspinatus and infraspinatus muscles receive innervation from the upper trunk via the suprascapular nerve. The lateral cord innervates the biceps and coracobrachialis muscles via the musculocutaneous nerve. The medial cord innervates cutaneous structures and the ulnar nerve.1,2,6
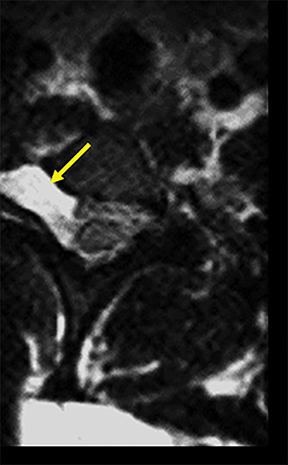
Tracing the peripheral nerves back to the spinal level, the C5 root is responsible for supraspinatus, infraspinatus, and the deltoid; C6 is responsible for biceps; C7 for forearm extensors and triceps; with C8 and T1 providing innervation for finger flexors and the intrinsic muscles of the hand. The normal MR anatomy of the brachial plexus is included in Figure 2.
A practical MR imaging protocol
The goal of imaging the BP is to visualize the entire course of the neural network from its preganglionic segments (eg, nerve rootlets and intraforaminal nerve segments) and the postganglionic segments from the dorsal root ganglion (DRG) to the terminal branches. Focused multiplanar, multisequence MRI is required to completely image the brachial plexus and offers superior contrast resolution compared to computed tomography (CT).4,6,7,8 Additionally, scanning coverage should include the C4-T2 levels for pre-fixed (C4-C8) and post-fixed (C6-T2) BP anatomic variation, and extend out to the axilla to include the proximal terminal branches.
Preganglionic and postganglionic segments
The preganglionic segments include the cervical spinal cord root entry zone of the ventral and dorsal nerve rootlets, the intrathecal course of these rootlets, and their intraforaminal course up until the DRG. Three-dimensional T2-weighted techniques using steady state free procession (SSFP) can help visualize the intrathecal and intraforaminal segments with high contrast and spatial resolution.9
The normal orientation of the BP (medial-superior to lateral-inferior) makes traditional sagittal, axial and coronal images difficult to interpret, as the plane of imaging is not aligned with the course of the brachial plexus structures.4 Consequently, oblique sagittal and coronal sequences are preferred techniques to capture the anatomy of the brachial plexus and its relationship to critical surrounding structures.1,4,10 In the setting of unilateral injury, bilateral coronal T2 sequences provide effective comparison between the affected side and the normal contralateral side2. Smaller field of view, unilateral sagittal sequences are preferable to evaluate for edema, enlargement of the segments of the brachial plexus, or enhancement if contrast is administered.1
Gadolinium-based IV contrast is not required for routine BP evaluation. However, if infection, trauma or neoplasm is a primary consideration, contrast should be administered to delineate the extent of involvement and to assess for potentially drainable collections.1,2
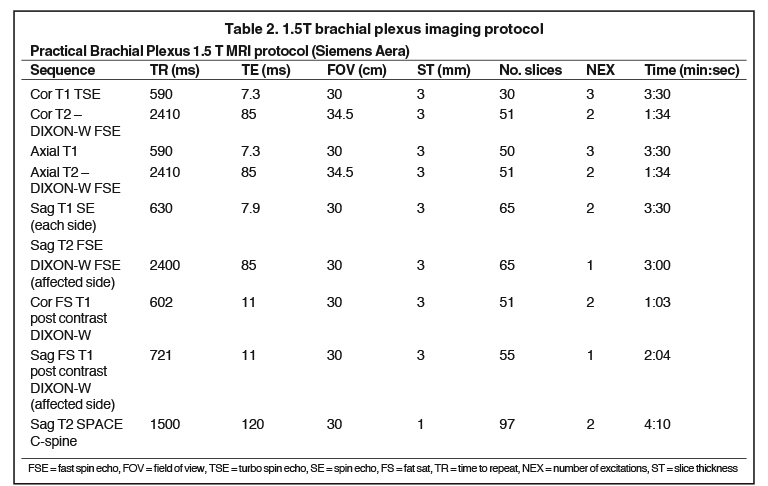
Tables 1 and 2 include practical brachial plexus MR protocols on 3T and 1.5T scanners, with the final two sequences (post-contrast) considered optional based on indication. Additional sequences, such as heavily T2-weighted 3D imaging with steady state free procession (SSFP) can also be performed if visualization of intradural nerve roots / myelographic images are desired.9 Of note, 1.5 Tesla (T) is the minimum required field strength for diagnostic quality images of the brachial plexus, with 3T being preferred secondary to improved signal to noise and contrast to noise ratios. Imaging accuracy, however, has been shown to be comparable between 1.5T and 3.0T systems7. Imaging can be performed with a surface coil such as a neurovascular array with multichannel phased array architecture or a wrap-around body surface coil in order to improve the signal to noise ratio.4,6
Common presenting symptoms
Presenting symptoms referable to the BP can range from vague and nonspecific (eg, regional shoulder pain, upper extremity weakness, or altered upper extremity/shoulder sensorium) to symptoms with specific nerve distribution (eg, pain or motor/sensory deficit). More specific symptoms such as scapular winging (due to long thoracic nerve injury), diaphragmatic dysfunction (involvement of the phrenic nerve C3-C5), or Horner’s syndrome (postganglionic C8-T1 involvement) can also be presenting complaints.2
Pathology categorization
Disease processes affecting the brachial plexus can be subdivided into the following broad categories: traumatic injury and several nontraumatic subtypes, including: infection, inflammatory brachial neuritis/neuropathy, benign or malignant neoplasms, radiation-induced plexopathy, vascular abnormalities, and compression of the plexus.
Traumatic injury
Perhaps the most significant utility of brachial plexus MR in the setting of trauma is to differentiate pre and post ganglionic injury, a distinction that has significant management implications.8,11,12 Identification of a preganglionic injury can be difficult and often requires recognition of a combination of direct (eg, high resolution 3D MRI or CT myelography) and indirect imaging characteristics.
Direct signs
High-resolution 3D T2- and CT myelography images can show anatomical continuity or lack thereof of intradural nerve rootlets.5,6,9,12 The course of the rootlets should be followed from the root entry zone to the DRG in the neural foramen. In the setting of a structurally intact yet injured nerve root, post contrast sequences may show abnormal enhancement of the injured nerve root relative to the control side.5,9,14
Indirect signs
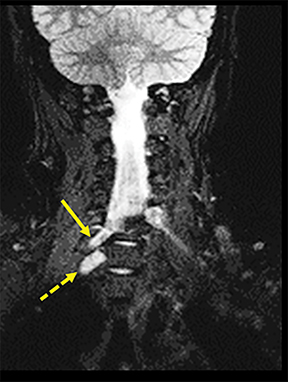
A traumatic pseudomeningocele may be present on T2-weighted images. The pseudomeningocele will usually show invagination into the affected neural foramen, with or without the detached nerve root within it (Figure 3). Contralateral deviation of the spinal cord is another indirect sign. Normally, the nerve rootlets anchor the spinal cord in the middle of the thecal sac, much like the taut ropes attached to large tents. Avulsion of nerve rootlets results in unopposed traction by the contralateral, intact nerve rootlets. Postganglionic traumatic injuries can demonstrate focal edema (hyperintense T2 signal) involving any part of the plexus distal to the DRG, anatomic discontinuity with or without clumping/retraction, or a peri-plexus hematoma.1,6,9,13
Infection
Infection of the brachial plexus can arise from a variety of sources, but it usually spreads directly from an adjacent structure such as an extension of spinal osteomyelitis, empyema/pulmonary parenchymal infections, glenohumeral septic arthritis, overlying soft tissue infection, or iatrogenic introduction of pathogens.2,15,16 Imaging characteristics of brachial plexus infections are similar to infectious processes elsewhere in that they result in T2 hyperintense edema, variable enhancement pattern (non-mass like), surrounding soft tissue inflammation and presence or absence of a demonstrable source collection.4,6,13,17,18
Inflammation
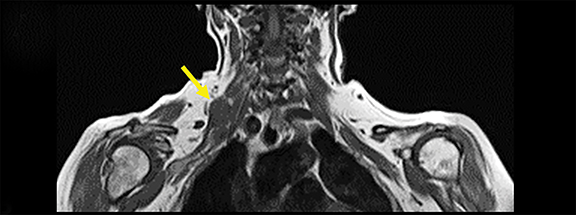
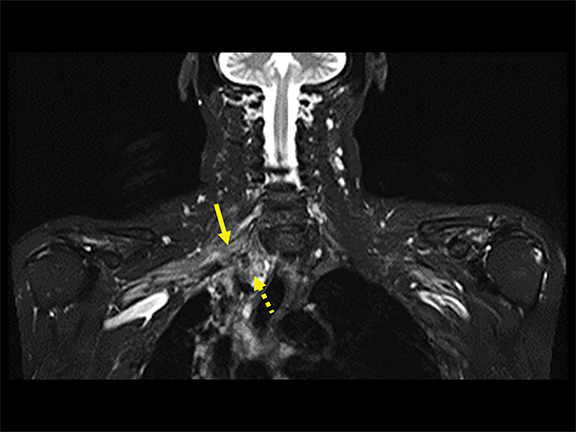
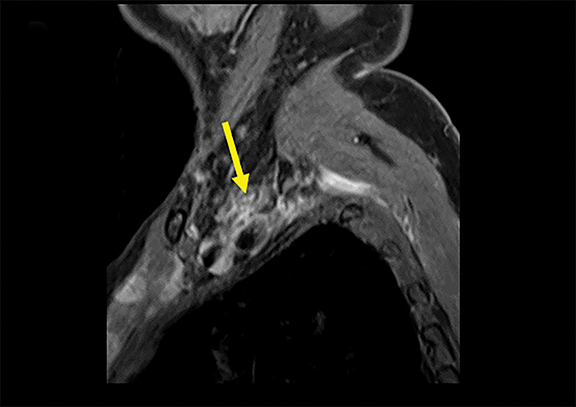
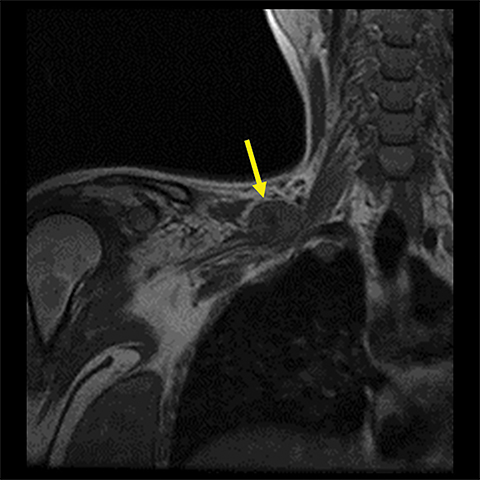
Spontaneous brachial plexitis, also known as Parsonage Turner Syndrome, typically presents with the constellation of spontaneous acute severe burning shoulder pain, subsequent sensory disturbance, and delayed weakness and atrophy.19, 20 The inflammatory processes involving structures adjacent to the brachial plexus can also secondarily involve the brachial plexus. The imaging characteristics of brachial plexus inflammation are nonspecific but consistent with inflammatory conditions elsewhere in the body. Commonly encountered features include T2 hyperintensity, thickening and variable enhancement of brachial plexus components, as well as enlargement of the affected shoulder girdle muscle with enhancement and hyperintense T2 signal (signs of acute/subacute denervation) (Figure 4).1,2,6,8,13
Benign neoplasms
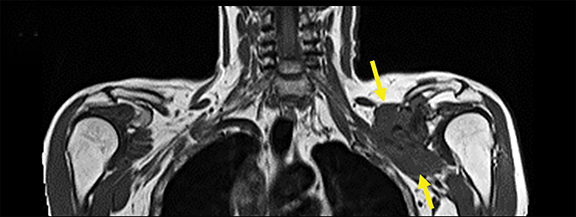
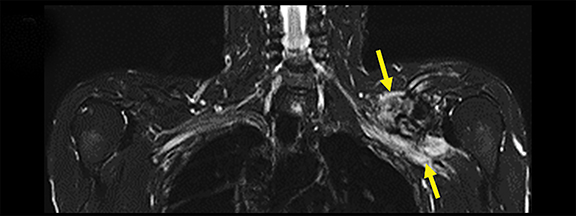
A variety of benign lesions can involve the brachial plexus. Specifically, these entities include fibromatosis, proliferative fasciitis, lipoma, hemangioma, brachial cleft cyst, lymphangioma, and benign neural and nerve sheath neoplasms (Figure 5).2 Fibromatosis (extra-abdominal desmoid) (Figure 6) and proliferative fasciitis can both present as rather large masses involving the brachial plexus, with the former usually presenting as painless, and the latter as exquisitely tender.21-24 Both of these lesions typically demonstrate T1 isointensity to surrounding muscle/soft tissue, heterogenous T2 hyperintensity, with mild enhancement in the case of proliferative fasciitis and avid enhancement in fibromatosis.23,24
Lesions such as lymphangiomas, lipomas, brachial cleft cysts, and hemangiomas occur rarely in the BP and demonstrate similar imaging characteristics as they would in other areas of the body.25 Lipomas are usually easily discernible from nonfatty lesions, as they characteristically demonstrate T1 and T2 hyperintensity, with signal dropout on fat-saturated sequences. One potential pitfall is differentiating benign lipoma from low-grade liposarcoma. In the setting of large lesions (>10 cm), significant non-fatty components, numerous septae, or heterogenous enhancement, PET-CT can be performed for better characterization.26,27
Benign nerve sheath tumors have been described in a plethora of prior publications. Briefly, they are commonly observed as T2 hyperintense lesions in the neural foramen, sometimes resulting in expansion and osseous remodeling.1,4,5,25
Malignant neoplasms
Malignant lesions can either primarily arise within the brachial plexus or spread to the brachial plexus secondarily. Primary malignant lesions involving the brachial plexus are predominately sarcomatous (low grade sarcoma, radiation induced sarcoma, osteosarcoma, Ewing Sarcoma, leiomyosarcoma, liposarcoma).2,21 These soft tissue masses often demonstrate overlapping imaging characteristics and can be difficult to differentiate based on imaging alone. Primary mesothelioma, malignant nerve sheath tumors and involvement from primary vertebral tumors such as chondrosarcoma or chordoma are also rarely seen involving the brachial plexus.2,21
In regards to metastatic disease, the most common primary malignancies are breast, lung, lymphoma, and head/neck cancer.2 Lung adenocarcinoma typically secondarily involves the brachial plexus via direct extension in the setting of a Pancoast tumor involving the superior sulcus (Figure 7). Breast carcinoma (Figure 8), lymphoma, and head/neck malignancies usually involve the brachial plexus via metastatic regional lymphatic spread2,25. In the setting of primary or metastatic brachial plexus involvement it is important to determine presence or absence of leptomeningeal enhancement/spread, relation to the ipsilateral vertebral artery, and extent of nerve root involvement.
Radiation plexopathy
Radiation plexopathy typically manifests as T2 hypointense thickening of the brachial plexus components without focal mass (Figure 9).1 In the setting of prior malignancy and local radiation to the brachial plexus, it is crucial for the clinician to attempt to differentiate tumor progression/recurrence from benign radiation-induced plexopathy changes. Time course is key to discerning these entities, as radiation induced plexopathy occurs between 5 and 30 months post radiation therapy (peak incidence 10-20 months post radiation).4, 6, 8, 13, 25 Furthermore, details of clinical presentation can help aid in diagnosis. For example, increasing/new pain or new Horner syndrome are more likely to reflect tumor recurrence/progression; while unilateral edema or parasthesia is more likely to reflect radiation-induced plexopathy.1
Compression of the brachial plexus
The neurovascular bundle can be compressed at several areas along the brachial plexus (Figure 10), resulting in a clinical constellation of symptoms commonly referred to as thoracic outlet syndrome. Particularly, the brachial plexus components can be affected at the interscalene triangle, costoclavicular space, or less commonly, the pectoralis minor space. Clinically, this syndrome can result in ulnar distribution hand weakness, hand/arm/neck pain/parasthesias, and upper extremity muscle atrophy.22 Symptomatology is often exacerbated/reproducible by arm raise. The syndrome is typically caused by anatomic variants such as a cervical rib, prominent lower cervical transverse processes, posttraumatic fibrous bands, or pectoralis muscle hypertrophy.28 MRI can be used to identify any of the aforementioned causative factors, and should include provocative testing in order to reproduce symptomatology during the time of the scan.2,22
Vascular abnormalities
A variety of space-occupying vascular abnormalities can result in brachial plexus compression, including but not limited to pseudoaneurysm, arteriovenous fistula (AVF), or arteriovenous malformation (AVM).2 The involved vessels include the subclavian, axillary, common carotid, and vertebral arteries. A variety of predisposing conditions can result in these lesions, which are best characterized on dedicated vascular studies such as CT angiography, MR angiography, and/or conventional angiography.
Conclusion
The brachial plexus can be efficiently imaged and effectively interpreted by the general radiologist when approached from a practical standpoint. Optimization of a practical BP imaging protocol is paramount to identify normal anatomy and associated pathology. Practical and useful information that can help the referring physician include, pre- vs. post-ganglionic location of lesion, mass vs. non-mass like enhancement, laterality or bilateral nature of disease, location of injury/mass/abnormality in BP segments (eg root, trunk, division, etc.), and anatomical region and surrounding structures involved (eg, interscalene space, costoclavicular triangle, relationship to subclavian/axillary vessels).
References
- Rehman I, Chokshi FH, Khosa F. MR Imaging of the Brachial Plexus. Clin Neuroradiol. 2014 ;24(3):207-216.
- Mikityansky I, Zager EL, Yousem DM, et al. MR Imaging of the Brachial Plexus. Magn Reson Imaging Clin N Am. 2012 (20): 791–826.
- Torres C, Mailley K, Del Carpio O’Donovan R. MRI of the Brachial Plexus: modified imaging technique leading to a better characterization of its anatomy and pathology. Neuroradiol J. 2013;26(6):699-719.
- Bowen BC, Pattany PM, Saraf-Lavi E, et al. The brachial plexus: normal anatomy, pathology and MR imaging. Neuroimaging Clin N Am. 2004;14(1):59-85.
- Van Es HW, Bollen TL, Heesewijk HPM. MRI of the brachial plexus: a pictorial review. Eur J Radiol. 2010;74(2):391–402.
- Martinoli C, Gandolfo N, Perez MM, et al. Brachial plexus and nerves about the shoulder. Semin Musculoskeletal Radiol. 2010;14(5):523–546.
- Tagliafico A, Succio G, Neumaier CE, et al. MR imaging of the brachial plexus: comparison of 1.5 T and 3 T imaging, preliminary experience. Skeletal Radiol. 2011;40 (6) 717-724.
- Sureka J, Cherian RA, Alexander M, et al. MRI of brachial plexopathies. Clin Radiol. 2009;64(2):208–218.
- Yoshikawa T, Hayashi N, Yamamoto S, et al. Brachial plexus injury: clinical manifestations, conventional imaging findings and the latest imaging techniques. Radiographics. 2006;26:S133–43.
- Demondion X, Herbinet P, Boutry N, et al. Sonographic mapping of the normal brachial plexus. AJNR Am J Neuroradiol. 2003;24(7):1303–1309.
- Van Es HW. MRI of the brachial plexus. Eur Radiol. 2001;11(2):325–336.
- Doi K, Otsuka K, Okamoto Y, et al. Cervical nerve root avulsion in brachial plexus injuries: magnetic resonance imaging and comparison with myelography and computerized tomography myelography. J Neurosurg. 2002;96(3 Suppl):277–284.
- Amrami KK, Port JD. Imaging the brachial plexus. Hand Clin. 2005;21(1):25–37.
- Hayashi N, Yamamato S, Okubo T, et al. Avulsion injury of cervical nerve roots: enhanced intradural nerve roots at MR imaging. Radiology. 1998;206(3):817–822.
- White HD, White BA, Boethel C, et al. Pancoast’s syndrome secondary to infectious etiologies: a not so uncommon occurrence. Am J Med Sci. 2011;341(4):333–336.
- Miron D, Bor N, Cutai M, et al. Transient brachial palsy associated with suppurative arthritis of the shoulder. Pediatr Infect Dis J. 1997;16(3):326–327.
- Ayoub T, Raman V, Chouwdhry M. Brachial neuritis caused by Varicella-Zoster diagnosed by changes in brachial plexus on MRI. J Neurol. 2010;257(1):1–4.
- Gerevini S, Mandelli C, Cadioli M, et al. Diagnostic value and surgical implications of the magnetic resonance imaging in the management of adult patients with brachial plexus pathologies. Surg Radiol Anat. 2008;30(2):91–101.
- Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973–978.
- Helms CA, Martinez S, Speer KP. Acute brachial neuritis (Parsonage-Turner syndrome): MR imaging appearance—report of three cases. Radiology. 1998;207(1):255–259.
- Saifuddin A. Imaging tumours of the brachial plexus. Skeletal Radiol. 2003;32(7):375–387.
- Aralasmak A, Karaali K, Cevikol C, et al. MR imaging findings in brachial plexopathy with thoracic outlet syndrome. AJNR Am J Neuroradiol. 2010;31(3):410–417.
- Wang CP, Chang YL, Ko JY, et al. Desmoid tumor of the head and neck. Head Neck. 2006;28(11): 1008–1013.
- Ludemann W, Dorner L, Tatagiba M, et al. Brachial plexus palsy from nodular fasciitis with spontaneous recovery: implications for surgical management. Case illustration. J Neurosurg. 2001;94(6):1014.
- Wittenberg K, Adkins M. MR imaging of nontraumatic brachial plexopathies: frequency and spectrum of findings. Radiographics. 2000;20(4):1023–1032.
- Kransdorf MJ, Bancroft LW, Peterson JJ, et al. Imaging of fatty tumors: Distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99–104.
- Suzuki R, Watanabe H, Yanagawa T, et al. PET evaluation of fatty tumors in the extremity: possibility of using the standardized uptake value (SUV) to differentiate benign tumors from liposarcoma. Ann Nucl Med. 2005;19(8):661–670.
- Demondion X, Herbinet P, Van Sint Jan S, et al. Imaging assessment of thoracic outlet syndrome. Radiographics. 2006;26(6):1735–1750.
Citation
A V, FH C. MRI of the brachial plexus: A practical review. Appl Radiol. 2016;(4):9-18.
May 1, 2016