MR imaging of breast implants: Useful information for the interpreting radiologist
Images
The principal purposes of MR implant imaging are to obtain information regarding implant integrity, to evaluate for post-implantation complications, and to evaluate the breast tissues for disease, namely breast cancer. This article will discuss multiple issues regarding breast implants that could be valuable to the interpreting radiologist, but it will principally focus on implant integrity evaluation.
Background on breast augmentation
Breast augmentation is performed for elective cosmetic enhancement, correction of congenital malformations, or reconstruction following breast surgery, principally mastectomy. The FDA has approved breast implants for breast augmentation in women at least 22 years of age and for breast reconstruction for women of any age.1 More than 3.5 million women in the United States have breast implants.2 For the past decade, it has been the most commonly performed cosmetic surgical procedure in the United States annually with 290,224 such procedures performed in 2013. 72% of these used silicone implants, and the remaining 28% used saline. Nearly 70% of these patients were under the age of 40. The expenditure for this procedure alone was over a billion dollars in 2013.3
From 1992 to 2006 the FDA restricted the use of silicone breast implants in the United States due to safety concerns regarding free silicone in the body and a possible association with connective tissue diseases and breast cancer.4 It was later determined that no scientific evidence existed of such a relationship.5, 6
Numerous materials (including direct silicone injection) and devices have been used for breast augmentation/reconstruction but are uncommon in the United States and are not a focus of this paper. Additionally, oncoplastic breast reconstruction techniques using autologous myocutaneous flaps and implanted tissue expanders are not a focus of this paper; however, it occurs commonly enough to briefly discuss it.
A tissue expanding device is placed within the mastectomy surgical bed in order to stretch the overlying skin in preparation for a breast implant placement at a later time. The breast expander is typically sequentially filled with saline over several appointments. The port on the side of the expander is designed to allow for serial percutaneous needle access for saline to be injected without rupturing the expander. The injection port is commonly surrounded by a magnetic marker which allows for percutaneous identification of the port at subsequent clinic appointments without requiring imaging localization. Until MRI compatibility has been confirmed, the metallic or magnetic component of this device should be considered a contraindication to MRI due to possible overheating, tissue expander displacement, and possible demagnetization of the localization marker.7 MRI-compatibility of implanted devices can be checked at http://www.MRIsafety.com.
Breast augmentation and cancer risk
Breast augmentation does not increase the risk of breast cancer or decrease survival rates of women with breast cancer;7 however, the mammographic evaluation of the implant-augmented breast is slightly more tedious than those without implants, given more images to review (implant and implant-displaced views), the associated tissue distortion, postsurgical change, and difficulty in evaluating the tissues on the lateral and deep margins of the implant. While such issues may reduce the mammographic sensitivity of cancer detection in the implant-augmented breast8 the sensitivity of breast MRI in the detection of breast cancer does not appear to be reduced.7 It is interesting to note that the presence of implants may facilitate detection of breast cancer on clinical breast exam.9
Despite the reduced sensitivity of mammography and sonography in the augmented breast, there is no recommendation for periodic contrast-enhanced MRI screening in this population, unless the patient would otherwise meet recommendations for MRI screening based on high risk factors for breast cancer.7 However, contrast-enhanced breast MRI has been reported to be of benefit in the detection of recurrent breast cancer in patients with implant reconstructed breasts.7,10 Additionally, given the reduced sensitivity of mammography and sonography in the augmented breast, when a patient presents for diagnostic evaluation of a palpable abnormality and no significant abnormality is discovered, then contrast-enhanced MRI should be strongly considered to further evaluate for tumor.8 Intravenous contrast is not necessary for implant integrity evaluation but is needed for neoplastic evaluation.
In January 2011 the FDA issued a warning regarding a possible association between breast implants and anaplastic large cell lymphoma (ALCL).11 The summary of findings stated that a possible association existed between breast implants and ALCL; however, reported it to be a rare finding. Additionally they reported that it was not possible to identify an association with a particular type of implant (silicone versus saline) or an association with the reasons for implant placement (reconstruction versus cosmetic augmentation). The association is more frequent with textured outer shell implants rather than smooth outer shell implants; however, accurate documentation of the outer shell texture reporting was questioned. The true cause of anaplastic large cell lymphoma with breast implants was uncertain. In most cases the lymphoma cells were found within the periprosthetic effusion contained within the fibrous capsule. No lymphomatous invasion was demonstrated beyond the fibrous capsule. Further information regarding this, and other useful information regarding implants, can be found on the FDA website (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/).
Silicone implant types
Five implant generations are commonly discussed in the literature.12 The most recent adaptations have incorporated various densities of cohesive viscosity silicone gel. Increased gel viscosity has the benefit of maintaining implant contour, may have a lower incidence of leakage, and is less likely to collapse despite shell integrity. Disadvantages of cohesive gel implants include a less supple feel of the breast versus non-cohesive silicone gel and contour deformity if the implant rotates within the implant pocket. The 3rd and 4th generation implants began using textured shells with the benefit of less capsular contracture and less implant migration.13 Despite advances in breast implants, shell integrity/implant rupture remains a clinical question that is often best answered with imaging.
MRI technique for the implant-augmented breast
MR imaging of the breast is best performed allowing relaxation of the tissues by imaging in the prone position and using a dedicated breast coil. Magnetic resonance imaging of the breast should be performed on a high-field strength magnet (at least 1.5 Tesla) due to its ability to better emphasize or reduce signal intensity from silicone, water, or fat.
STIR silicone-selective sequences will demonstrate hyperintense silicone with water suppression. Silicone-saturated images will give hyperintense water signal with silicone suppressed. Turbo spin echo T2 weighted images are also very useful in evaluation of the breast containing a silicone implant. Examining the implant shell using a cross-referencing tool across axial, sagittal, and coronal planes is very useful for evaluating contiguity of radial folds versus discontinuous elastomer shell.
Intravenous contrast with a gadolinium-based contrast agent is unnecessary when evaluating solely the integrity of the breast implant. Intravenous gadolinium contrast should be added if enhancement characteristics of the adjacent tissues are needed to evaluate for neoplasm.7
MR Imaging of the augmented breast
It is beneficial for the radiologist to know both common and uncommon imaging signs suggestive of implant rupture, the composition of the implants being imaged, surgical approach used (axillary, subareolar, inframammary, umbilical), history of prior breast implants, and time since placement or revision. Knowing the surgical approach and temporality can help differentiate probable post-surgical changes versus other breast pathology. Knowing location of initial implant plane placement can help identify herniation due to loss of tissue integrity adjacent to the implant, such as overlying pectoralis muscle, and help explain contour deformities and/or pain. It is important to know the type of implant being imaged (single versus dual lumen), and the composition of each, in order to determine expected versus abnormal findings related to the implant and adjacent tissues. Knowing prior implant history may help to avoid a false conclusion of implant rupture in the rare setting of a patient who has undergone implant revision and has residual silicone in the soft tissues from the previous silicone implants. Surgical history is also important when evaluating for complications of breast augmentation, which would include hematoma and infection, among other postoperative findings.14
The 5th edition of the ACR BI-RADS Atlas 201315 provides a systematic outline for MRI evaluation of breast implants with description of implant material and lumen type (saline, silicone, other materials, single-lumen versus multilumen), implant location (retroglandular versus retropectoral), abnormal implant contour (focal bulge), intracapsular silicone findings (radial folds, subcapsular lines, keyhole signs, linguini sign), extracapsular silicone (breast, lymph nodes), water droplets, and peri-implant fluid.
The breast implant location is typically described in relation to the pectoralis major muscle and is either in the prepectoral/subglandular location (anterior to the pectoralis major muscle), or subpectoral (posterior to the pectoralis major muscle).
Variations of the implant appearance on MRI without rupture can appear as 1) Intact implant with uninterrupted fibrous capsule; 2) Intact implant with a small amount of periprosthetic fluid within the fibrous capsule; 3) Intact implant with radial folds; or 4) Intact implant with adjacent calcification and capsular thickening.
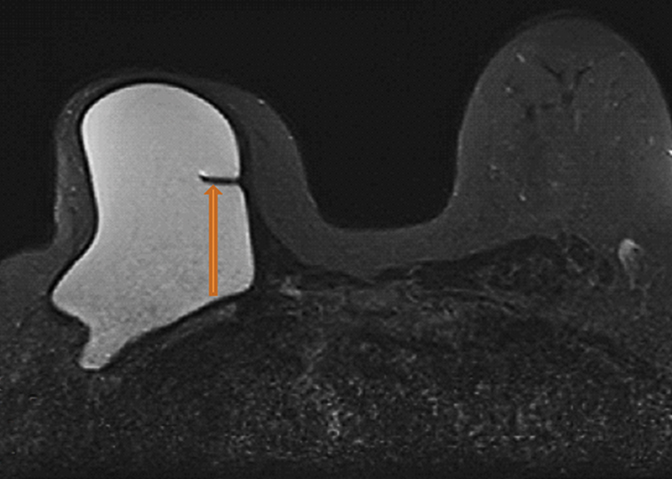
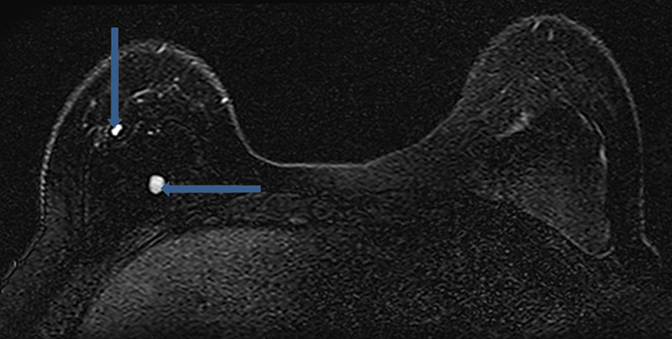
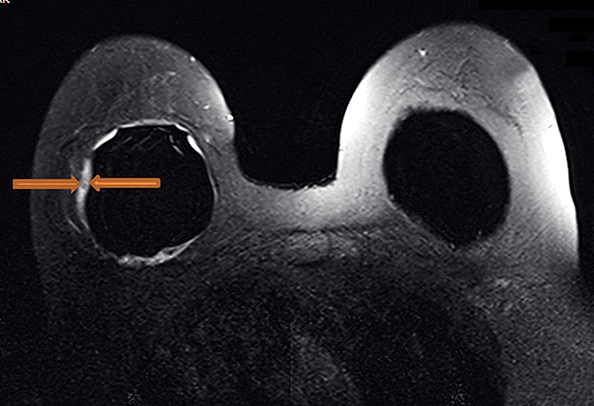
Radial folds, as shown in Figure 1, or shell invaginations, are one of the most common causes for false positive findings on MRI for implant shell rupture. On close inspection silicone should be retained within the implant. Radial folds and periprosthetic fluid should be considered variations of normal following breast implant placement. Radial folds are also commonly found with capsular contraction and subsequent reduction of the surgical bed volume. With violation of the capsule or overlying musculature (subpectoral implants), herniation of the implant into the adjacent parenchyma can occur and result in a contour deformity without necessary implant rupture.
Complex radial folds differ from radial folds because they are multidirectional and the contiguity of the silicone elastomer shell may be difficult to trace. Complex radial folds are considered a normal variant; however, more thorough evaluation for early implant rupture should be performed when they are present. This finding makes the exclusion of an early implant rupture difficult or impossible.
Implant rupture
The clinical diagnosis of implant rupture based on physical exam findings is unreliable and will miss more than half of implant ruptures, especially when loss of volume or cosmetic deformity are absent.12 While breast pain on physical exam is a strong predictor of implant rupture, the most common symptom is contour deformity (44%), followed by implant displacement (20%), mass formation (17%), pain (13%), and inflammation (3%).12 Loss of substance from the implant is most commonly related to spontaneous loss of shell integrity and rarely attributable to a traumatic event.16 Most implant ruptures occur 10-15 years following placement with an increasing incidence over time17. Average incidence of rupture is two ruptures per 100 implant years. It is estimated that 98% of implants will be intact after 5 years and 83-85% after 10 years.18 - 21 In a safety update released by the FDA in 2011, it states that 20% of women with implant augmentation will need explantation within 10 years of placement and an astounding 50% of patients with implants for breast reconstruction will require explantation within 10 years of placement.22
Magnetic resonance is the imaging gold standard for evaluation of integrity of the implant shell secondary to high spatial resolution and excellent contrast between the implants and the adjacent soft tissues. The ability to suppress or enhance silicone, as well as suppressing signal intensity of water and fat, aids in providing the highest sensitivity and specificity for implant rupture among all imaging modalities. MRI has a respective sensitivity and specificity for rupture between 80-90% and 90-97%. Reported rates of implant rupture detection with MRI are between 78 and 89% and only 25-30% with mammography.23-25A secondary benefit of MRI is the lack of ionizing radiation. Specialized imaging tools with MRI can also be used, such as calculation of implant volume26, 3-D rendering with intraluminal evaluation of the implant shell27 and MR spectroscopy for the detection of extra-mammary silicone.28
The two main categories of breast implant rupture are intracapsular implant rupture and extracapsular implant rupture. An intracapsular rupture occurs when the integrity of the implant shell is violated with leakage of silicone into the space adjacent to the implant without extending beyond the fibrous capsule created by the patient’s postsurgical response. An extracapsular rupture is defined by free silicone extending beyond the fibrous capsule and into the soft tissues of the breast. An intracapsular rupture is obligate with an extracapsular rupture and is consequently more common than extracapsular rupture, an obvious statistical assessment.
“Gel bleed” is a term used for microscopic silicone leakage through a presumably intact implant shell. This has been explained to be a due to the chemical affinity of the silicone gel for the silicone elastomer of the implant shell resulting in a transudation of the silicone gel through the implant shell.14 More distant extracapsular silicone (axillary or more distant sites) can be seen in the setting of gel bleed. The search for extracapsular silicone is best done using STIR sequences with silicone enhancement and searching for focal areas of high signal intensity. Once extracapsular silicone is present within the body, it can reach distant locations and has been reported in the liver, inguinal lymph nodes, pleural fluid, and synovium. A “siliconoma” can be palpable and sometimes painful and is the result of an immune response to the silicone. Distant silicone could be due to implant rupture versus gel bleed.
A related concept is “rupture without collapse” and was reported by Berg et al in 1995 to describe a ruptured implant without characteristic findings indicating rupture, principally a collapsed implant shell.29 With the increased use of textured implants, “rupture without collapse” will likely increase as a proportion of implant ruptures, given that the fibrous capsule is more adherent to the implant shell.30
Signs of possible implant rupture12,31
There are several indications of possible implant rupture:
- Contour deformity within the implant resulting in a bulging border. This has been described as a “rat-tail sign” when this bulging border is pronounced.
- Indistinct border of the implant or an irregular margin. This could be seen with calcification of the fibrous capsule.
- Variations in signal intensity in the silicone gel can occur when mixed with water or serum, presumably from implant integrity loss with infiltration from bodily fluids. These variations in silicone gel signal intensity can be diffuse or focal. When focal, this finding is referred to as a “salad oil sign” or “droplet sign.” It is important to note that this finding can be “normal” post implant placement without apparent rupture as occasional small droplets of water or small amounts of air can be identified within the silicone implant. In this circumstance, more subtle signs of implant rupture should be sought.
- MRI imaging signs of “keyhole,” “teardrop,” or “noose” will demonstrate increased signal intensity at the end of a radial fold on the silicone sensitive sequences and will be outlined by the invaginated implant shell. In the setting of an intact implant shell, the small extraluminal area outlined by the shell invagination will be dark on the silicone sensitive sequences and likely demonstrate increased signal intensity on fluid sensitive sequences.
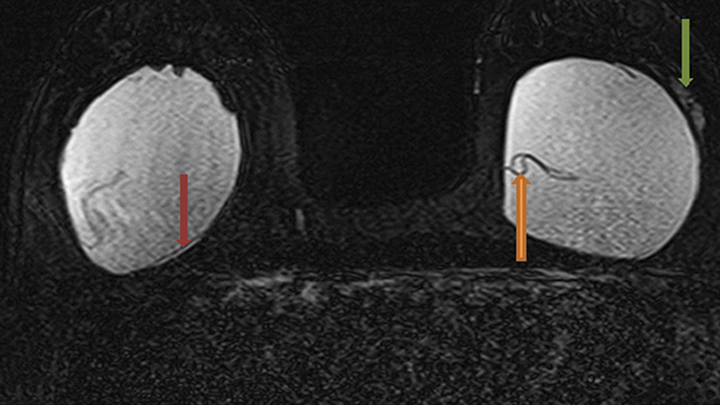
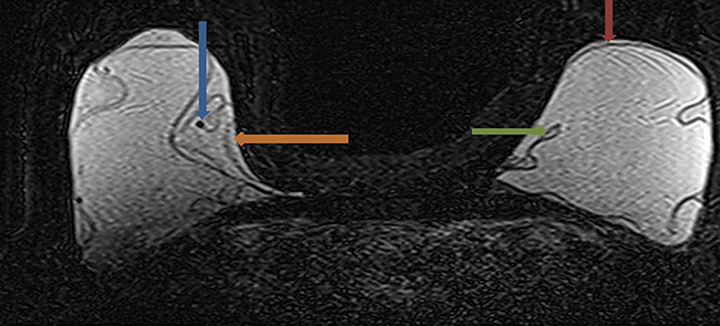
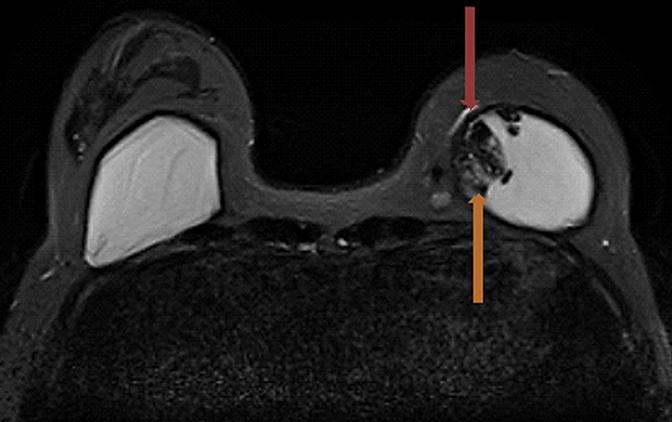
Signs of definitive implant rupture12, 31 (Figures 2A-E)
Similarly, there are several indications of definitive implant rupture:
- Subcapsular lines. These are distinct hypointense lines (bordered by silicone signal intensity on both sides) running parallel to the fibrous capsule but are otherwise contiguous with the implant shell and represent intracapsular rupture.
- Free silicone. This indicates violation or loss of integrity of the capsular shell with extravasation of silicone into the adjacent tissues.
- Linguine sign. Multiple low signal intensity curvilinear lines are present floating within the silicone gel and represent collapse of the silicone elastomer implant shell. As the name implies, these have an appearance similar to linguine noodles.
- Railroad track sign. Paired parallel subcapsular lines in the silicone gel.
The most specific MRI finding for intracapsular silicone implant rupture is the “linguine sign” and was first described by Gorczyca, et al, in 1992.32 In a study by Vestito, et al,33 the “linguine sign” was the single most frequently identified finding with intracapsular rupture. They reported a sensitivity of 96%, specificity of 77% and diagnostic accuracy of 90%. They also reported that neither the “noose sign” nor the “droplet sign” were statistically significant findings for rupture, unless combined with other signs indicative of rupture. The “subcapsular line sign” is a similar finding that likely represents an earlier variant of the “linguine sign.”
The most common reason for having a false-positive MRI indicating implant rupture occurs when a single sign of implant rupture is used to make the diagnosis, including the most specific finding, “linguine sign.”33
Standardized scoring system for implant integrity
Maijers, et al31 recommended utilization of a standardized scoring system for implant integrity using the term “SI-RADS,” Silicone Implant Reporting and Data System. This system assesses two separate features: integrity of the implant and extracapsular leakage of silicone.
The scoring system in each category ranges from 0 to 4. In keeping with the BI-RADS numbering system, a “0” represents an incomplete examination with additional imaging required. Regarding implant integrity, one and two are “intact” and “probably intact,” respectively with no further clinical management recommended. Three and four indicate “probably ruptured” and “ruptured” with recommendation for referral for further evaluation.
Extracapsular leakage of silicone was similarly numbered with “0,” representing an incomplete study with the recommendation for additional imaging. One and two represent “no extracapsular leakage” and “probably no extracapsular leakage” without recommendation for further management. Three and four represent “probable extracapsular leakage” and “definite extracapsular leakage” with recommendation for referral for further evaluation and management. An additional note was made that in circumstances of a “0” assessment or incomplete, additional imaging or a second opinion from a radiology colleague should be sought in an attempt to more definitively categorize the findings.
Imaging exam considerations
Factors to consider when deciding which imaging modality to use for evaluation of implant integrity include clinical relevance of the information obtained, existent contraindication to MRI, availability of the imaging modality, the radiologist’s ability to adequately interpret the imaging acquired for implant integrity, and the cost of the exam. While the principle use of MRI in the setting of the augmented breast is to evaluate implant integrity and is certainly more sensitive for detection of rupture, this expensive imaging modality is not always necessary, especially in the circumstances of mammographically or sonographically evident extracapsular ruptures. A responsibility of the radiologist is to provide guidance to the clinician/surgeon regarding which imaging study, if any, is best for answering the clinical question.
Chung, et al34 developed an imaging algorithm for which modality to use in evaluation of implant rupture. The pretest probability of rupture in asymptomatic patients was reported at 7%. In this population of asymptomatic women with a negative screening sonogram, the probability of rupture was less than 2%. If the patient was asymptomatic but the sonogram was positive for signs of rupture, then the probability of rupture increased to 38%. A positive MRI evaluation in the same population (asymptomatic with positive sonogram) increased the likelihood of an implant rupture to 86%.
In patients with symptoms suggestive of implant rupture and an implant age of less than 10 years, only 31% were ruptured. If further sonographic evaluation is negative for implant rupture in the symptomatic patient, then the probability of rupture decreased to 16%. If sonogram demonstrated signs consistent with implant rupture, then probability increased to 80%. If MRI evaluation was added to the symptomatic patient and indicated rupture, then probability of rupture was 98%.34
In the symptomatic patient with implants greater than 10 years of age, the pretest probability of implant rupture was 64%. If sonographic signs were present indicating implant rupture, then probability of rupture increased to 94%.34 In this setting the addition of MRI would find few additional implant ruptures not detected by sonography.
While the FDA recommends follow-up MRI scanning biannually, starting in the third year after implant placement, the positive predictive value of such imaging in the asymptomatic population is likely not cost effective. MRI imaging of breast implants is best reserved for answering the question of implant rupture with ambiguous mammographic or sonographic findings. Certainly, if the implant integrity is in question, MRI is a more accurate imaging modality for evaluating the extent of silicone leakage into the adjacent tissues or axilla. Improvements in imaging technology and newer generations of breast implants may result in different implant rupture detection rates.
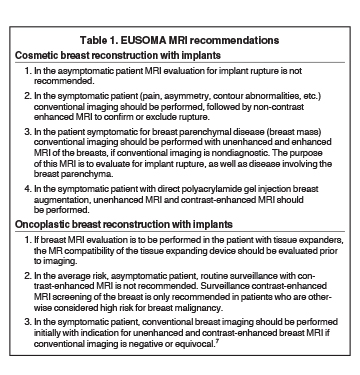
In this author’s opinion, EUSOMA recommendations7 for MR imaging of the implant augmented/reconstructed breast are concise and provide a reasonable framework to guide imaging decisions (Table 1).
Other post-implantation findings
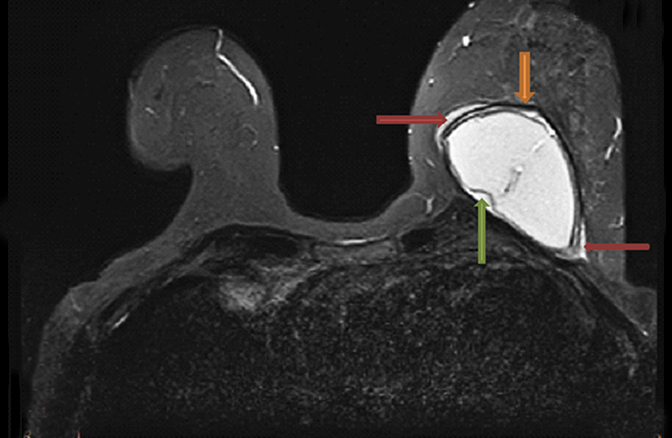
Part of the normal healing response following breast augmentation is development of a thin fibrous capsule around the implant. In circumstances when the fibrous response is exuberant, capsular contraction resulting in discomfort and poor cosmesis can occur and is one of the most common complications following implantation. It occurs more commonly with subglandular (8.6%) versus subpectoral (2.8%) and more commonly with smooth shell versus textured implants.13 Capsular contracture is primarily a clinical diagnosis, and while imaging of the fibrous response itself is difficult, consequent morphologic changes can generally be seen.
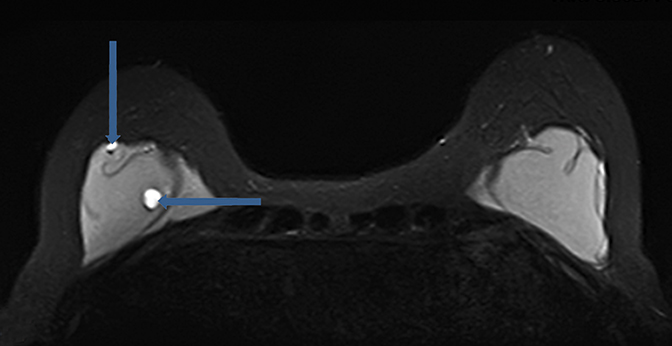
While peri-implant fluid (Figure 3) can be a physiologic reaction to implantation, consideration of adjacent hematoma or infection should also be considered with additional imaging sequences performed as necessary for further characterization.
Postimplant complications which have been more recently described, and appear to be more closely related to textured implants, are the development of a double capsule and late seroma formation.35 In the instance of double capsule, chronic inflammation/infection and the outer biofilm are possible etiologies.36 Late seroma was defined as periprosthetic fluid collection but occurred greater than one year following breast augmentation and is included in an extensive differential diagnosis of periprosthetic fluid collection to include hematoma, infection, implant rupture, synovial metaplasia, inflammation, double capsule, cancer, and other idiopathic causes. The treatment for this periprosthetic fluid collection generally consists of antibiotic therapy, ultrasound-guided aspiration, or surgery. An interesting finding in this series was that neither infection nor malignancy was implicated as the cause for late periprosthetic fluid collection. The clinical management of this late postoperative complication is for removal of the textured implants with immediate replacement with a smooth round silicone implant.36
Conclusion
This paper has discussed some of the principle issues regarding imaging evaluation of the implant-augmented breast with a primary focus on evaluation of implant shell integrity. Discussions regarding association between augmentation and breast cancer, types of implants, MRI technical aspects, normal and abnormal findings in the augmented breast, imaging and clinical factors regarding implant rupture, imaging exam considerations, and other post-implantation findings have been discussed and are important factors to consider in order for the radiologist to provide the best service to the patient and referring providers. The most current product safety information and other updates regarding breast implants can be found on the FDA website (http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/).
References
- Center for Devices and Radiological Health U.S. Food and Drug Administration. FDA Update on the Safety of Silicone Gel-Filled Breast Implants. June 2011. http://www.fda.gov/downloads/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM260090.pdf
- Zambacos GJ, Molnar C, Mandrekas, AD. Silicone lymphadenopathy after breast augmentation: case reports, review of the literature, and current thoughts. Aesthetic Plast Surg. 2013;37(2) 278-289.
- American Society of Plastic Surgeons. 2013 Plastic Surgery Statistics Report. http://www.plasticsurgery.org/Documents/news-resources/statistics/2013-statistics/plastic-surgery-statistics-full-report-2013.pdf)
- Food and Drug Administration, Center for Devices and Radiological Health. Guidance for Industry and FDA Staff - Saline, Silicone Gel, and Alternative Breast Implants. November 17, 2006. U.S. Department of Health and Human Services. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm071228.htm
- Bondurant S, Ernster V, Herdman R, eds. Safety of Silicone Breast Implants. Washington, D.C: National Academy Press; 1999.
- Tugwell P, Wells G, Peterson J, et al. Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel. Arthritis Rheum. 2001;44(11):2477-2484.
- Sardanelli F, Boetes C, Borisch B, et al. Magnetic resonance imaging of the breast: Recommendations from the EUSOMA working group. Eur J Cancer. 2010; 46(8):1296-1316.
- Stoblen F, Rezai M, Kummel S. Imaging in patients with breast implants-results of the First International Breast (Implant) Conference 2009. Insights Imaging. 2010;1(2):93-97.
- Handel N. The effect of silicone implants on the diagnosis, prognosis, and treatment of breast cancer. Plast Reconstr Surg. 2007; 120(Suppl 1):81S-93S.
- Heinig A, Heywang-Kobrunner SH, Viehweg P, et al. Value of contrast medium magnetic resonance tomography of the breast in breast reconstruction with implant. Radiologe. 1997; 37(9):710-717.
- Anaplastic Large Cell Lymphoma (ALCL) In Women with Breast Implants: Preliminary FDA Findings and Analyses. http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm239996.htm
- Juanpere S, Perez E, Huc O, et al. Imaging of breast implants-a pictorial review. Insights Imaging. 2011; 2(6):653-670.
- Namnoum J, Largent J, Kaplan H, et al. Primary breast augmentation clinical trial outcomes stratified by surgical incision, anatomical placement and implant device type. J Plast Reconstr Aesthet Surg. 2013;66(9): 1165-1172.
- Yang N, Muradali D. The augmented breast: a pictorial review of the abnormal and unusual. AJR Am J Roentgenol. 2011; 196(4):W451-60.
- BI-RADS Committee. ACR BI-RADS Atlas. 5th ed. Reston, VA: American College of Radiology; 2013.
- Kleinmeyer AE, Mahoney MC. MRI for Breast Implant Evaluation. In: Kuhl C, Mahoney MC. Breast MRI: Expert Consult: Online. London: Elsevier Health Sciences: 2013:140-151.
- Amano Y, Aoki R, Kumita S, et al. Silicone-selective multishot echo-planar imaging for rapid MRI survey of breast implants. Eur Radiol. 2007; 17(7):1875-1878
- Holmich LR, Fryzek JP, Kjoller K, et al. The diagnosis of silicone breast-implant rupture: Clinical findings compared with findings at magnetic resonance imaging. Ann Plast Surg. 2005; 54(6):583-589.
- Holmich LR, Friis S, Fryzek JP, et al. Incidence of silicone breast implant rupture. Arch Surg. 2003; 138(7):801-806.
- Holmich LR, Vejborg I, Conrad C, et al. The diagnosis of breast implant rupture: MRI findings compared with findings at explantation. Eur J Radiol. 2005; 53(2):213-225.
- Holmich LR, Vejborg IM, Conrad C, et al. Untreated silicone breast implant rupture. Plast Reconstr Surg. 2004;114(1):204-142;discussion 215-216.
- FDA provides updated safety data on silicone gel-filled breast implants. Agency highlights information women should know when considering implants. FDA News Release. June 22, 2011. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm260235.htm
- Cher DJ, Conwell JA, Mandel JS. MRI for detecting silicone breast implant rupture: meta-analysis and implications. Ann Plast Surg. 2001; 47(4):367-380.
- Hölmich LR, Vejborg I, Conrad C. The diagnosis of breast implant rupture: MRI findings compared with findings at explantation. Eur J radiol. 2005;53(2):213-225.
- Herborn CU, Marincek B, Erfmann D, et al. Breast augmentation and reconstructive surgery: MR imaging of implant rupture and malignancy. Eur Radiol. 2002; 12(9):2198-2206.
- Rudolph R, Forcier N. Calculation of silicone breast implant volumes using breast magnetic resonance imaging. Aesthet Surg J. 2009; 29(4):310-313.
- Moschetta M, Telegrafo M, Capuano G, et al. Intra-prosthetic breast MR virtual navigation: a preliminary study for a new evaluation of silicone breast implants. Magn Reson Imaging. 2013; 31(8): 1292-1297.
- Pfleiderer B, Heindel W. MRI and MR spectroscopy after silicone breast implants in the female breast. Rofo. 2001; 173(7):571-579.
- Berg WA, Caskey CI, Hamper UM, et al. Single- and double- lumen silicone breast implant integrity: prospective evaluation of MR and US criteria. Radiology. 1995; 197(1):45-52.
- Calobrace MB, Hammond D. Anatomic gel implants: from concept to device. Plast Reconstr Surg. 2014; 134(3 Suppl):4S-9S.
- Maijers MC, Niessen FB, Veldhuizen JF, et al. MRI screening for silicone breast implant rupture: accuracy, inter- and intraobserver variability using explantation results as reference standard. Eur Radiol. 2014; 24(6):1167-1175.
- Gorczyca DP, Sinha S, Ahn CY, et al. Silicone breast implants in vivo: MR imaging. Radiology. 1992; 185(2):407-410.
- Vestito A, Mangieri FF, Ancona A, et al. Study of breast implant rupture: MRI versus surgical findings. Radiol Med. 2012; 117(6):1004-1018.
- Chung K, Greenfield M, Walters M. Decision-analysis methodology in the work-up of women with suspected silicone breast implant rupture. Plast Reconstr Surg. 1998; 102(3):689-695.
- Bengston B, Brody GS, Brown MH, et al. Late periprosthetic fluid collection after breast implant working group. Managing late periprosthetic fluid collections (seroma) in patients with breast implants: The consensus panel recommendation and review of the literature. Plast Reconstr Surg. 2011; 128(1): 1-7.
- Lista F, Tutino R, Khan A, et al. Subglandular breast augmentation with textured, anatomic, cohesive silicone implants. Plast Reconstr Surg. 2013; 132(2): 295-303.
Citation
Kist JWHSCDPOK.MR imaging of breast implants: Useful information for the interpreting radiologist. Appl Radiol. 2015; (10):18-24.
October 3, 2015