Medulloblastoma
Images


Case Summary
A toddler was brought to the emergency room for vomiting and a bulging anterior fontanelle.
Imaging Findings
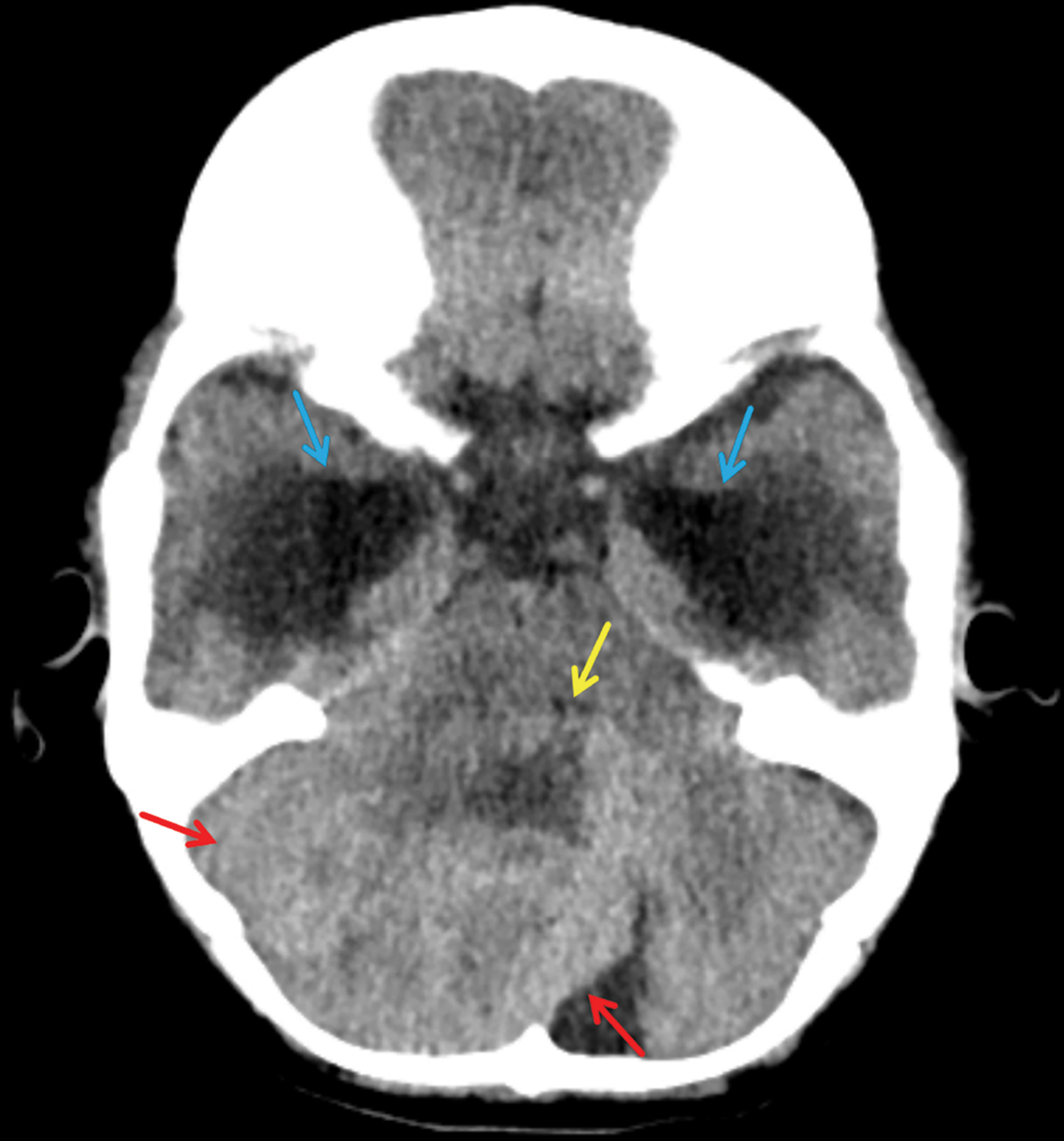
Noncontrast CT demonstrated a heterogeneous, hyperattenuating posterior fossa mass compressing the 4th ventricle anteriorly. This caused obstructive supratentorial hydrocephalus with interstitial flow of CSF (Figure 1).
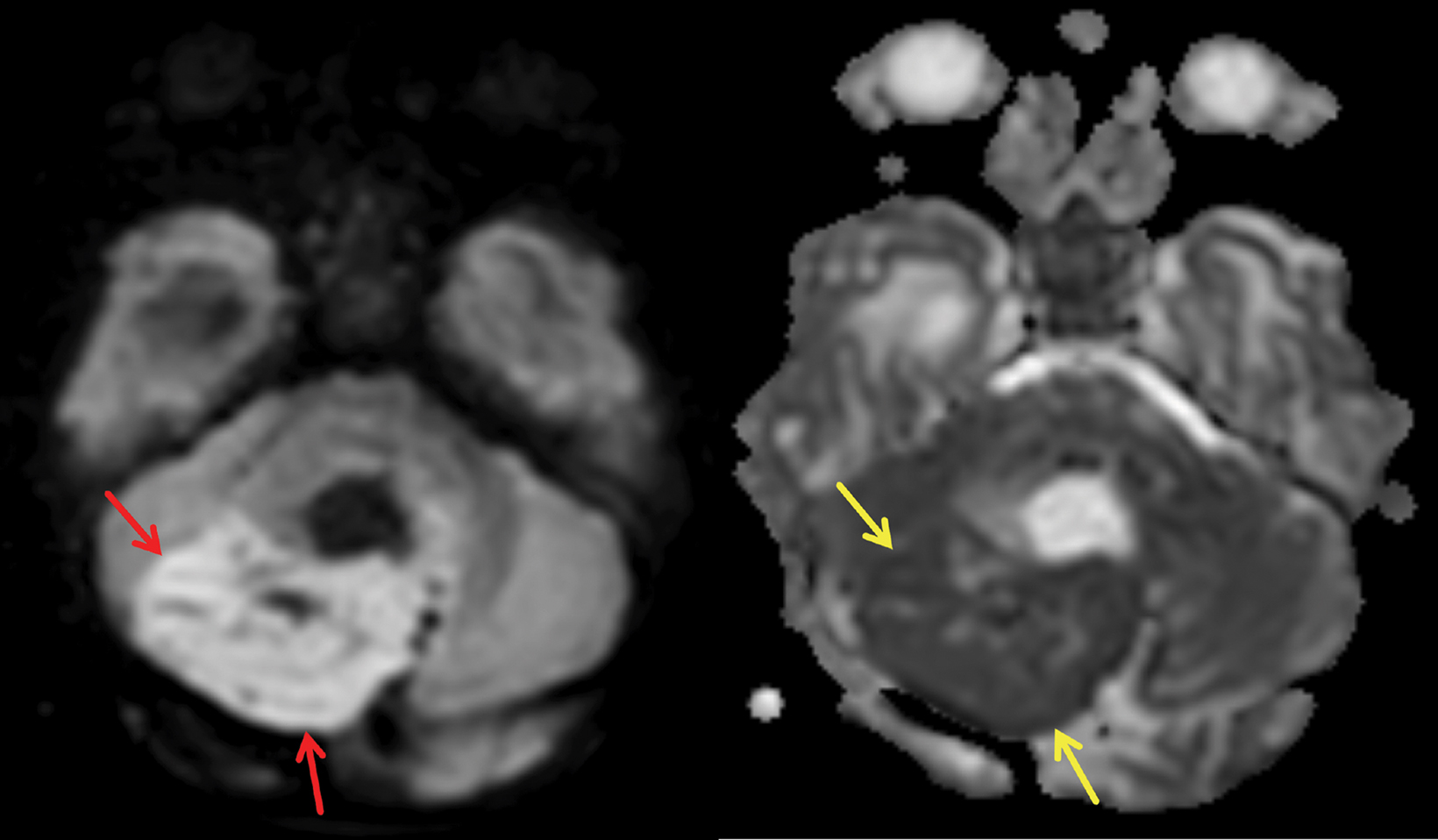
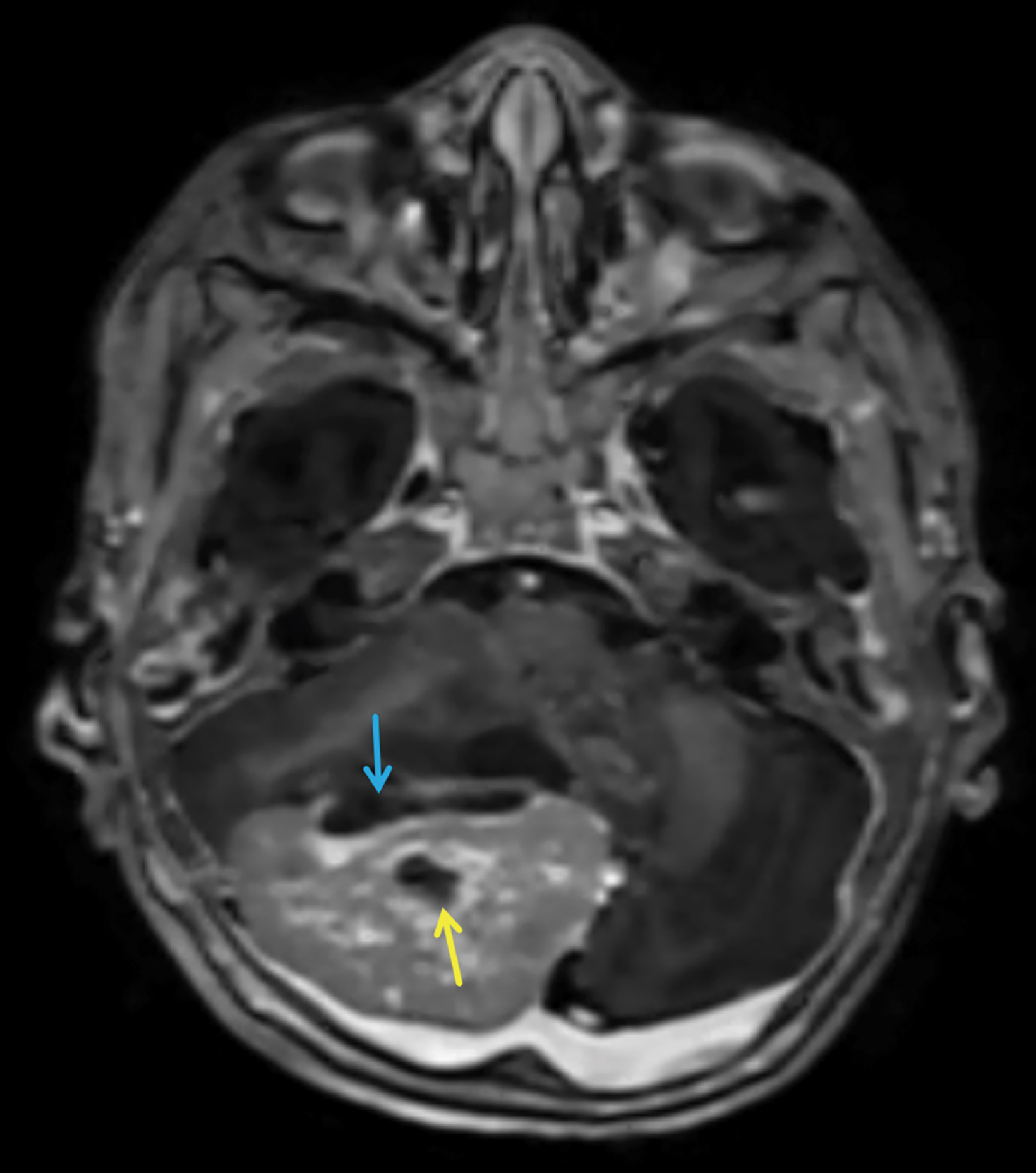
Brain MRI subsequently demonstrated the mildly T2 hyperintense mass to be centered in the medial aspect of the right cerebellar hemisphere with local mass effect, including compression of the fourth ventricle and brainstem (Figure 2). The solid portions of the mass showed restricted diffusion (Figure 3). Marked lateral ventricular enlargement was present with increased signal intensity in the adjacent periventricular white matter corresponding to transependymal flow of CSF. The mass was heterogeneously enhancing following gadolinium administration (Figure 4).
Diagnosis
Medulloblastoma. Differential diagnosis includes ependymoma, astrocytoma, and atypical teratoid rhabdoid tumor.
Discussion
Medulloblastomas (MB) are a subtype of CNS embryonal tumors that arise exclusively in the posterior fossa and are the most common malignancy in the pediatric population, especially males, with a peak incidence around the age of 8 years. A well-circumscribed, hyperintense, round mass arising in the area of the cerebellar vermis, hemispheres, and fourth ventricle with impairment of CSF flow in male pediatric patients, is typical of MB. The incidence of MB begins to fall after childhood and is rarely seen in patients over age 40.1
Historically, MBs have been defined histologically. This anatomic approach to classification does not, however, influence the approach to therapy. Recently, MBs have been characterized using molecular subtyping, which has led to the creation of four additional categories to consider: WNT activated (WNT), sonic hedgehog activated (SHH) — which can be TP53-wildtype or mutant — and Group 3 and 4 , which are tumors not related to WNT or SHH, also sometimes grouped as non-WNT/non-SHH tumors.2
Research into the molecular groupings of MBs demonstrate the heterogeneity of these tumors. SHH tumors have been linked to granular neuron precursor cells (GNP) of the cerebellar external granule cell layer. WNT tumors appear to arise from progenitor cells in the dorsal brainstem. Group 3 tumors may occur in GNPs but also in cerebellar ventricular zone cells or other progenitors, and Group 4 tumors, being the most heterogeneous of the groups, may arise from a variety of precursors.3 Molecular subtyping has been linked to patient outcomes and has been used to stratify patients into risk categories.2
On computed tomography, MBs typically are hyperattenuating midline vermian masses with contrast enhancement that obstruct the fourth ventricle.1 However, magnetic resonance imaging is the gold standard for evaluating pediatric MB. MRI should include T1, T2, and diffusion weighted imaging (DWI). The appearance of MB on T1 can range from hypo- to isointense, with masses on T2 ranging from hypo- to hyperintense.5 DWI is an excellent tool for discriminating different posterior fossa masses, with MBs showing increased restriction and diffuse hyperintense signal.5
Evidence suggests MB molecular subtypes display specific features on MRI. One feature, tumor location, appears to have a high positive predictive value for determining molecular subgroups.6 Perreault, et al, reported that WNT tumors often arise in the cerebellopontine angle cistern/cerebellar peduncle, SHH tumors (as is most likely in our case) most commonly arise in the cerebellar hemispheres, and Group 3 and 4 tumors arise within the vermis.6
Further investigation has expanded upon these findings by showing that, while all molecular subtypes may be extracerebellar, only SHH tumors can be completely intracerebellar.7 In addition, WNT, Group 3, and Group 4 tumors almost always invade the fourth ventricle.7 Currently, diagnosing MB molecular subtypes based on MRI is not always standard of care, but continued research, refinement of MRI protocols, and improved technology may create such a possibility.
Treatment is based on the presenting size, shape, and age of the patient, typically starting with surgical resection followed by combined radiation and chemotherapy. Imaging should include the entire neuroaxis to evaluate for leptomeningeal metastasis.
Post-treatment imaging vigilance, including diffusion weighed imaging must be maintained to assess for recurrent tumor8 and delayed leptomeningeal spread of disease.
Conclusion
Medulloblastomas are the most common pediatric malignant tumor and should be considered in any patient presenting with signs and symptoms of cerebellar dysfunction or intracranial hypertension. These tumors can be divided into molecular subgroups that influence treatment and, while not currently the standard of care, MRI may be beneficial to differentiate them.
Standard treatment consists of surgical resection followed by radiation and chemotherapy.
References
- Alli S, Isik S, Rutka JT. 11 posterior fossa and brainstem tumors in children. Principles of Neurological Surgery (Fourth Edition). 2018:183-203.e7. http://www.sciencedirect. com/science/article/pii/B9780323431408000111. doi: https://doi.org/10.1016/B978-0-323-43140-8.00011-1.
- Khatua S, Song A, Citla Sridhar D, Mack SC. Childhood medulloblastoma: Current therapies, emerging molecular landscape and newer therapeutic insights. Curr Neuropharmacol. 2018;16(7):1045-1058. Accessed Mar 6, 2020. doi: 10.2174/1570159X15666171129111324.
- Azzarelli R, Simons BD, Philpott A. The developmental origin of brain tumours: A cellular and molecular framework. Development. 2018;145(10):dev162693. http://dev.biologists.org/content/145/10/dev162693.abstract. doi: 10.1242/dev.162693.
- Gur RE, Kaltman D, Melhem ER, et al. Incidental findings in youths volunteering for brain MRI research. AJNR. Am J Neuroradiol. 2013;34(10):2021-2025. https://www.ncbi. nlm.nih.gov/pubmed/23811972. doi: 10.3174/ajnr.A3525.
- Eran A, Ozturk A, Aygun N, Izbudak I. Medulloblastoma: Atypical CT and MRI findings in children. Pediatr Radiol. 2010;40(7):1254-1262. Accessed Mar 6, 2020. doi: 10.1007/ s00247-009-1429-9.
- Perreault S, Ramaswamy V, Achrol AS, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR. Am J Neuroradiol. 2014;35(7):1263-1269. https://www.ncbi. nlm.nih.gov/pubmed/24831600. doi: 10.3174/ajnr.A3990.
- Colafati GS, Voicu IP, Carducci C, et al. MRI features as a helpful tool to predict the molecular subgroups of medulloblastoma: State of the art. Ther Adv Neurol Disord. 2018;11. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6024494/. Accessed Mar 6, 2020. doi: 10.1177/1756286418775375.
- Morana G, Alves CA, Tortora D, et al. Added value of diffusion weighted imaging in pediatric central nervous system embryonal tumors surveillance. Oncotarget. 2017;8(36):60401-60413. Published 2017 Jul 25. doi:10.18632/oncotarget.19553.
References
Citation
J W, J S, SA J, DJ A, CM S, RB T, AJ T. Medulloblastoma. Appl Radiol. 2022;(1):43-45.
January 6, 2022