Low-Intensity Ultrasound Makes Knees Stronger After Injury or Surgery
 National Institutes of Health (NIH) supported research at The University of Alabama in Huntsville (UAH) reports that low-intensity ultrasound therapies may one day rebuild stronger knees following injury or surgery.
National Institutes of Health (NIH) supported research at The University of Alabama in Huntsville (UAH) reports that low-intensity ultrasound therapies may one day rebuild stronger knees following injury or surgery.
Anu Subramanian, PhD, professor of chemical and materials engineering at UAH, a part of the University of Alabama System, has been the principal investigator in research exploring the effects of ultrasound on cartilage regrowth in NIH-funded in-vitro and bovine cadaver knee research since before she joined the university in 2018. Her total NIH research awarded so far in this field is $1,857,229.
Dr. Subramanian’s research has progressed over the years from the test-tube phase through cadaver cow knee work, rabbit testing and now to equine knee investigations in collaboration with the Colorado State University (CSU) equine model.
Cartilage that serves as a cushion between knee bones is a protein-rich matrix with very few cells. It has no blood vessels so it cannot regrow itself. Orthopedic surgeons try to make up for that with a technique that drills into the adjoining bone to create microfractures to produce blood rich in stem cells.
Some of the resulting mesenchymal cells convert to another type, chondrocyte cells that are needed to regrow collagen. But some instead become fibroblasts and create a much less sturdy form of collagen.
The result is a weaker bond and the potential for future failure where the injury or surgical intervention took place. Dr. Subramanian has been investigating a low-cost method that uses continuous low-intensity ultrasound (cLIUS) therapy to regrow quality post-operative cartilage in joints like the knee or shoulder.
Under the current four-year, $494,000 continuing R01-NIH grant that’s part of her total award of $1,957,903, ultrasound will be used following equine surgeries at CSU. The advancing research brings us closer to a time when an injured knee will undergo Magnetic Resonance Imagery and then a computer will tell the surgeon where and how best to apply post-operative ultrasound so that resulting cartilage regrowth has characteristics very close to the original material.
“We have demonstrated the proof-of-concept in a rabbit model, and currently we are working to translate the findings to a larger animal model, the equine model, which has joint characteristics close to that of a human,” Dr. Subramanian says.
“At CSU, Dr. Brad Nelson and Dr. Jeremiah Easley will be performing the surgeries in sheep and testing the ultrasound regimen to repair defects in sheep. They will also help evaluation of the repair using biomechanical and immunological techniques.”
Dr. Nelson is an assistant professor of equine surgery at CSU, while Dr. Easley is the co-director of the CSU Preclinical Surgical Research Laboratory and an assistant professor in the CSU Department of Clinical Sciences.
“I also hope to continue my collaboration with Dr. Hendrik Viljoen, distinguished professor in the Department of Chemical and Biomolecular Engineering at the University of Nebraska-Lincoln,” she says. “He and I started this project together at UNL.”
Dr. Sarma Rani, a UAH associate professor of mechanical and aerospace engineering, is a co-investigator in the current grant which also supports doctoral students Shahid Khan (biotechnology science), Aryana Singh Bhati (biotechnology), Sattik Basu (mechanical and aerospace engineering) and Owen Trippany (mechanical and aerospace engineering; chemical and materials engineering).
After first being approved by the Institutional Animal Care and Use Committee (IACUC) of the American Association for Laboratory Animal Science, earlier rabbit research was successful in demonstrating that ultrasound promotes integration of newly grown tissue with the surrounding native tissue, Dr. Subramanian says. That was further proof of concept in the stepped research paradigm used to advance technologies like ultrasound toward human use.
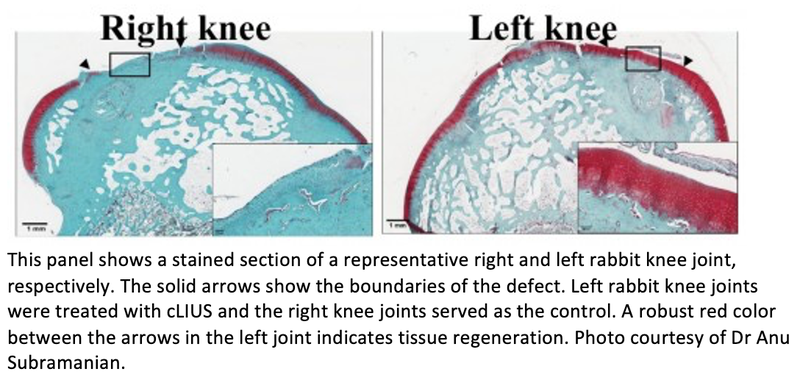
“Briefly, we surgically created defects in the knee joints of rabbits using a technique called microfracture, where a small defect is created and the cartilage is debrided and tiny holes are drilled into the underlying bone to harness the endogenous supply of mesenchymal stem cells that have healing potential,” she says.
“Our results demonstrate that healing of chondral defects treated with microfracture can be accelerated by employing the cLIUS regimen at the beneficial frequency,” Dr. Subramanian says. “We have demonstrated in our IACUC-approved study that joints that received ultrasound upon microfracture had improved cartilage repair and better repair scores when compared to control joints that did not receive any ultrasound, but underwent microfracture.”
The results also demonstrated that ultrasound can encourage repair in the initial pro-inflammatory joint environment that immediately follows surgery or injury, she says. “Inflammation during the recovery and regeneration phase plays a critical role in modulating the repair outcome,” Dr. Subramaian says.
“For example, during the early recovery phase, the joint environment harbors a plethora of cytokines moieties that, if left unchecked, can have a catabolic effect and impede the repair process. So, treatment modalities need to encourage healing and repair, but also mitigate the catabolic effects of the cytokines present. Our work has shown that continuous low intensity ultrasound, when used at the beneficial regimen, promotes repair and regeneration in an inflammatory environment.
In the rabbit research, the inflammation was created by the surgical process itself.
“From a very basic science perspective, we have shown that ultrasound can coax or encourage the multi-potent mesenchymal stem cells to adopt the lineage of cells that populate the cartilage and do so in a pro-inflammatory environment using very specific cellular control machinery,” she says.
Dr. Subramanian says she’s always been intrigued by the intersection of cross-cutting problems between biology, medicine and engineering.
“I think my engineering perspective brings quantitative aspects to this research, and I am able to define the underlying challenges using a process modeling approach,” she says. “Over the years, I have ventured more and more into cartilage biology and the underlying biochemistry and molecular biology aspects.”
She’s identified areas of new exploration during the research. “One of the most interesting problems that needs addressing is what happens to the joint and its constituent tissues immediately after joint trauma,” she says.
If the events after the initial insult remain unresolved, long-term joint damage may occur.
“In this NIH-funded project, I am addressing long-term cartilage repair,” she says. “I am now beginning to create ex-vivo (outside the living body) multi-tissue models to better understand the early events upon joint trauma, and that will remain my focus for the next five years.”
Related Articles
Citation
. Low-Intensity Ultrasound Makes Knees Stronger After Injury or Surgery. Appl Radiol.
December 9, 2022