Leptomeningeal Spread of Metastatic Wilms Tumor
Case Summary
A child with previously confirmed stage III Wilms tumor (WT) who underwent left nephrectomy and whole-abdomen radiation and who had received cycles of chemotherapy presented with multiple lung metastatic lesions 6 months after initial chemotherapy. He underwent resection of the lung lesions, and pathology confirmed WT relapse. Five months later, he presented to the emergency department with seizures and headaches. Cerebrospinal fluid (CSF) analysis obtained in the emergency department showed no abnormal findings.
Imaging Findings
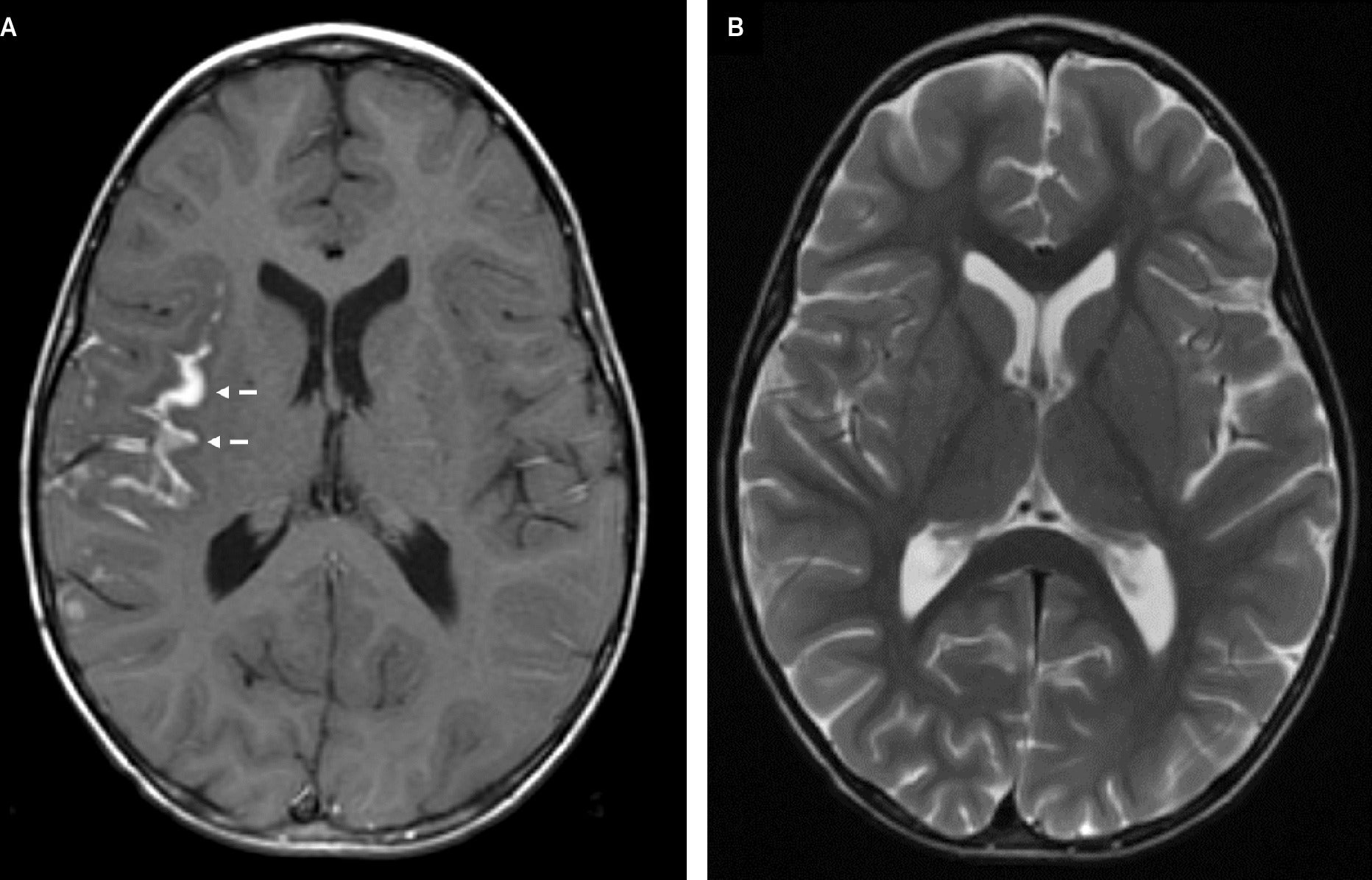
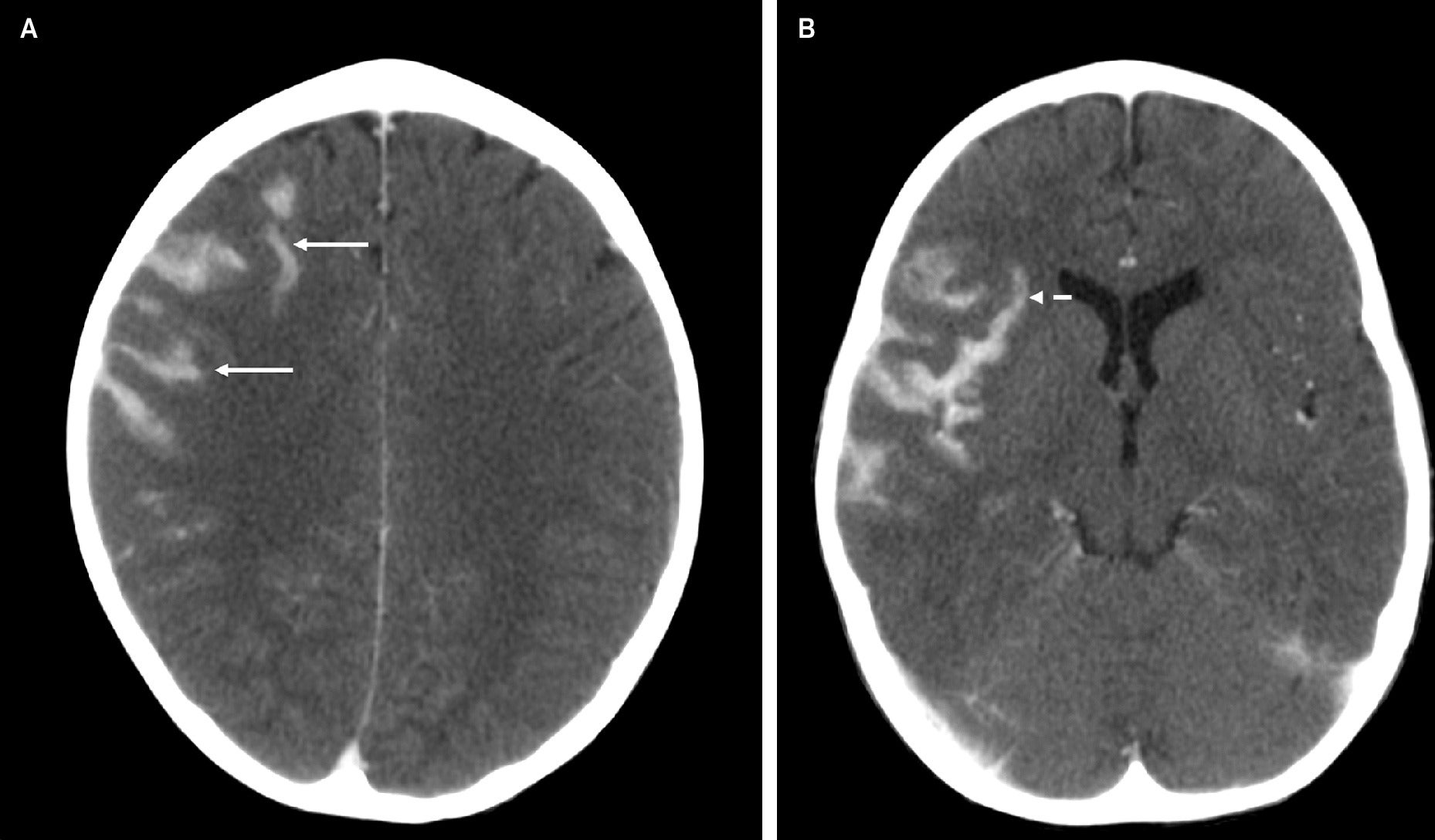
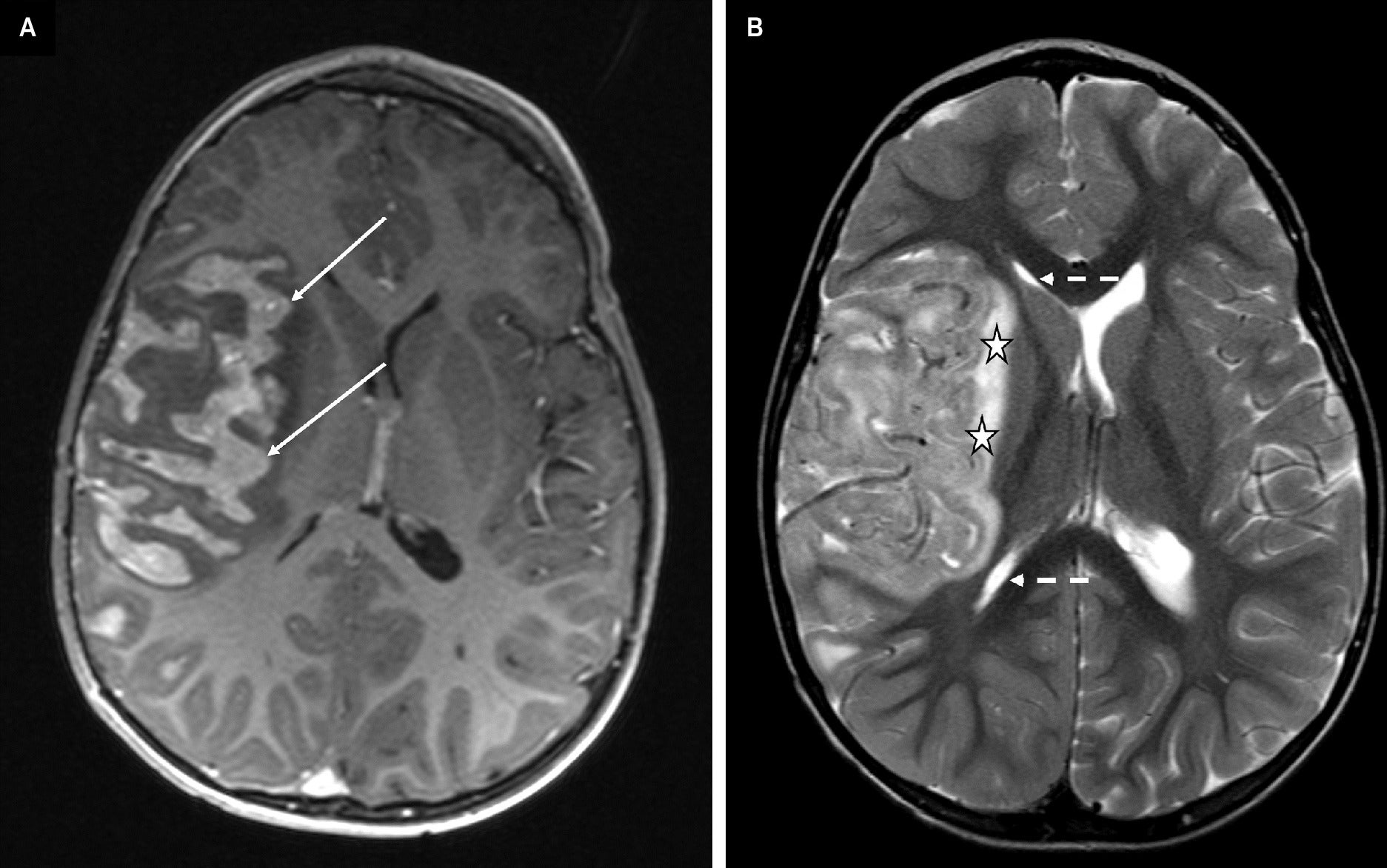
Cerebral magnetic resonance imaging (MRI) ( Figure 1 ) demonstrated signal abnormality in the Sylvian fissure along the insular cortex. No significant vasogenic edema was noted. Cranial computed tomography (CT) ( Figure 2 ) obtained 19 days later showed an interval increase in the extension of the hyperattenuating lesion in a gyriform distribution, at this time also involving the right frontal lobe, allowing for the differences in modalities between studies. A follow-up MRI obtained 7 days after the CT demonstrated a mass-like lesion with gyriform distribution, markedly increased size, and significant surrounding vasogenic edema, consistent with worsening leptomeningeal disease ( Figure 3 ).
A post-contrast axial gradient-echo T1-weighted image (A) demonstrates gyriform enhancement in the deep Sylvian fissure, most pronounced along the insular cortex (dashed arrows). The corresponding axial turbo spin echo T2-weighted image (B) demonstrates no significant surrounding parenchymal vasogenic edema. The lesion is not easily visible on this sequence.
Post-contrast axial brain CT images demonstrate new, mass-like hyperattenuation in the right frontal lobe (A, solid arrows) and more pronounced hyperattenuating lesions in the right Sylvian fissure, in a gyriform distribution along the insular cortex and frontotemporal operculum (B, arrows), compared with a brain MRI 19 days earlier.
Axial post-contrast gradient echo T1-weighted image (A) and axial turbo spin-echo T2-weighted image (B). A brain MRI performed one week after CT demonstrates rapid progressive disease with an enhancing mass-like lesion with a gyriform distribution occupying the sulci, consistent with leptomeningeal disease (A, solid arrows). Note the interval worsening of the right cerebral hemisphere edema seen with T2 signal of the underlying brain parenchyma (B, stars), partial effacement of the right lateral ventricle (B, dashed arrows), and leftward midline shift.
Diagnosis
Intracranial metastasis of WT.
Discussion
WT is the most common renal malignancy in children. 1 In the United States, approximately 500 new cases are diagnosed each year, most commonly in children <15 years old. WT typically presents as a solitary lesion; however, 7% of cases are multifocal and 5-9% are bilateral. 1 The tumor is caused by abnormal kidney embryogenesis due to mutations in genes such as WT1, CTNNB1, and WTX, which lead to the failure of intermediate mesoderm to properly transform into metanephric mesenchyme, a crucial step in the development of functional kidney cell types. However, these mutations only account for a small proportion of cases. 1 Some WT cases occur as part of multiple malformation syndromes, such as WAGR, Beckwith-Wiedemann, and Denys-Drash syndrome. 1 Prior to the advent of effective chemotherapy, intracranial metastases were found in up to 13% of cases at autopsy. However, with modern therapeutic protocols, the incidence of brain metastases has decreased significantly, ranging between 0.5% and 1%. 2 Leptomeningeal metastases from solid tumors can occur through various mechanisms, with hematogenous spread being the most common. 6
The reported case provides insight into the pathogenesis of metastatic WT to the brain. Early lesions may present as leptomeningeal nodular enhancing lesions along the cortical surface, which can rapidly evolve into large masses over time. Leptomeningeal metastases are rare in children and typically occur as a late complication of cancer progression or recurrence after current oncologic treatments that allow longer survival. 6,7 In this case, the patient presented with nonspecific neurological symptoms and CSF analysis did not demonstrate malignant cells. Consistent with the literature, this patient experienced relapse of intracranial disease several months to years following first-line treatment. 2-5
When reviewing the prior published cases of intracranial relapse of WT, 7-9 we found that only three prior patients in the literature had demonstrated intracranial metastasis in the form of leptomeningeal disease, confirmed by imaging. Porto et al described a patient with relapse 15 months after initial diagnosis. An MRI of the patient’s brain demonstrated a nodular enhancing lesion along the cortical surface in the left frontal lobe, which might represent leptomeningeal disease. 7 Takamiya et al showed head CTs from a 4-year-old patient who presented with generalized convulsion following nephrectomy for WT. 9 Initial CT demonstrated hyperattenuation along the sulci within the bilateral frontal lobes and left Sylvian fissure, which was interpreted as hemorrhage. Ten months later, numerous high-attenuation masses were noted bilaterally, which were later confirmed by biopsy as metastases of WT. In retrospect, we hypothesize that the hyperattenuating areas along the sulci in their case were likely an early manifestation of leptomeningeal disease, remarkably similar to our case. Madsen et al described a 4-year-old patient in whom MRI demonstrated a heterogeneous, multinodular, enhancing area in the left parietal and posterior frontal lobes, also consistent with leptomeningeal spread. 8 The remainder of the cases that we reviewed from the literature presented with intracranial masses rather than predominantly leptomeningeal involvement. While one may question whether these masses represent the result of enlargement and confluence of leptomeningeal disease, it is not possible to confirm this without early imaging. Furthermore, it is possible that the larger cohorts of intracranial relapse in WT patients may include individuals with leptomeningeal disease, rather than intra-axial masses. 2,3 However, detailed imaging analysis was beyond the scope of these prior studies.
Overall, intracranial relapse of WT remains a diagnostic dilemma. Due to the absence of standardized screening methods across different pathologies, these cases are likely to be underestimated. 6,7 However, early recognition is of utmost importance, as 5-year survival rates after intracranial recurrence may be as low as 28%. 2 In pediatric patients with leptomeningeal disease secondary to non-hematological, non-central nervous system malignancies, CSF analysis is not always helpful. As seen in our case, false-negative lumbar punctures are possible in pediatric patients with leptomeningeal disease. Porto et al demonstrated that only 11 out of 18 patients (61%) with brain MRI findings consistent with leptomeningeal metastases had cytologically positive lumbar puncture. 7 Additionally, the clinical presentation of these patients is nonspecific. 2,3
Conclusion
Intracranial metastasis is a rare but known complication of WT. Neuroimaging, particularly MRI, is crucial for evaluating cancer patients with neurological symptoms, as CSF cytology and clinical presentation lack specificity. With imaging, we observed leptomeningeal disease, an infrequent occurrence in WT documented in only 3 other cases in the literature. Our findings provide comprehensive imaging characteristics of leptomeningeal disease and contribute to the understanding of the pathogenesis of this rare presentation of intracranial relapse in WT patients.
References
Citation
Martinez-Correa S, Pollock AN, Teixeira SR.Leptomeningeal Spread of Metastatic Wilms Tumor. Appl Radiol. 2025;
doi:10.37549/AR-D-25-0104
October 1, 2025