Imaging “worst headache of my life” Part 1: Conditions in which the initial CT is often positive
Images


Headache is a common problem that results in over 2 million visits to the emergency department (ED) every year.1 Most headaches are benign, self-limiting conditions or manifestations of chronic headache syndromes.2 Even among patients presenting with the “worst headache of my life,” benign causes far exceed life-threatening causes.3
Nevertheless, several serious and potentially life-threatening etiologies of headache can and do cause patients to present to the ED. Physicians are tasked with differentiating the benign causes from the more serious, and imaging often plays a major role in making this distinction.
Noncontrast head computed tomography (NCT) is the most common initial imaging test ordered for headache patients presenting to the ED.1 In most cases, this initial NCT will be normal. However, several potentially life-threatening causes of headache do appear as abnormalities on NCT, and these manifestations can range from subtle to highly conspicuous. In light of the high prevalence of normal CT examinations for headache, maintaining vigilance when reviewing these studies can be a challenge. An active search pattern may help radiologists to avoid missing subtle but potentially serious diagnoses.
This 2-part series aims to review an approach to imaging patients presenting with severe headaches using a method that mirrors the everyday experience of radiologists. This first part focuses on potentially life-threatening diseases that commonly produce positive findings on the initial NCT. The goal of this article is to facilitate development of an active search pattern for NCT, which is encountered most frequently when evaluating headaches.
Part 2 will discuss diseases that often do not show findings on NCT. Because NCT may appear normal in these patients, these conditions require a heightened level of clinical suspicion to make the diagnosis, as well as a solid knowledge of the strengths and limitations of various imaging techniques. The goal of the second part is to explain how imaging modalities other than NCT may be used to review particular elements of the clinical presentation that may prompt radiologists to suggest such further imaging and make an accurate diagnosis.
Subarachnoid hemorrhage
Subarachnoid hemorrhage (SAH) is often the first diagnosis considered when evaluating a severe headache. However, most patients with this complaint do not have SAH. In one prospective investigation, 78% of patients presenting with the “worst headache of my life” did not have subarachnoid hemorrhage.4 Among unselected patients with headaches of varying severity who present to the emergency department, SAH is even less common, with several studies reporting an incidence of 1% or less.1,3,5
Despite these facts, SAH is a frequently pursued diagnosis because of its high rates of rebleeding and poor outcomes associated with untreated hemorrhage.6 Stated differently, the negative consequences of failure to detect SAH far outweigh the costs of screening every such patient. Perhaps because of the sheer volume of patients who present with headache, clinical misdiagnosis remains a problem, with initial misdiagnosis of SAH seen in 25% to 51% of cases.5 Even in the ED, one of every 20 cases of SAH may fail to be diagnosed.7
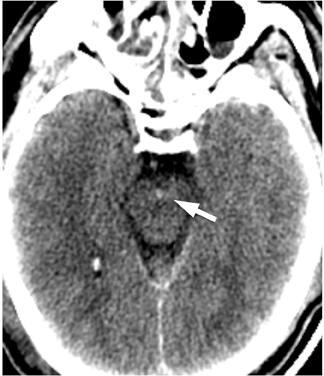
One literature review found CT to be highly sensitive for detecting SAH, with rates of 91% to 98% within the first 12 to 24 hours.8 Significantly, after 12 hrs to 24 hrs, CT’s sensitivity for SAH declined 82% to 84%, and the sensitivity of unenhanced CT at 1 week fell to 50%.8 Because of the potentially catastrophic consequences of missing SAH and the inability of imaging to detect all cases of SAH, lumbar puncture is still recommended in cases of clinically suspected SAH with a negative CT.6,9 Nonetheless, by focusing on certain imaging features on unenhanced CT, the diagnostic yield of unenhanced CT can be increased even several days after initial presentation. In particular, the dependent portions of the subarachnoid space and ventricular system should be carefully examined, as these areas may reveal subtle levels of SAH that have settled in the cerebrospinal fluid (CSF). Because patients are usually scanned in the supine position, particularly important locations include the interpeduncular cistern, the occipital horns of the lateral ventricles, the quadrigeminal plate cistern, and the dependent portions of the Sylvian fissures (Figure 1).
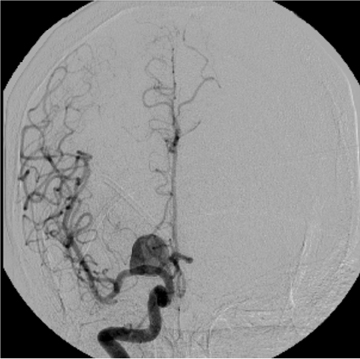
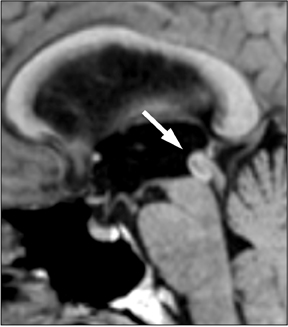
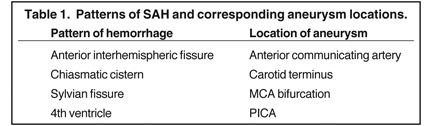
Once SAH is detected, the cause must be sought. In 80% of cases, the etiology is a ruptured aneurysm.10 In these patients, the pattern of hemorrhage on the initial NCT can help predict the site of the ruptured aneurysm, as indicated in Table 1.11 In some cases, the aneurysm itself can be seen as a filling defect against a background of subarachnoid blood (Figure 2). Imaging with conventional angiography, CT angiography (CTA), or less commonly, magnetic resonance angiography (MRA) is mandatory for definitively localizing the aneurysm.
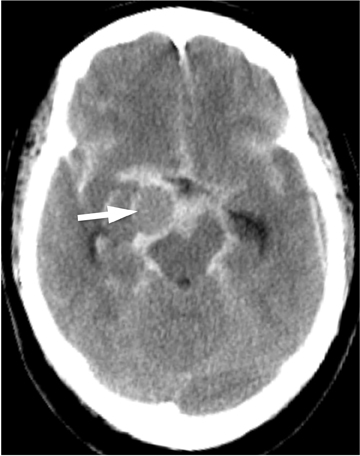
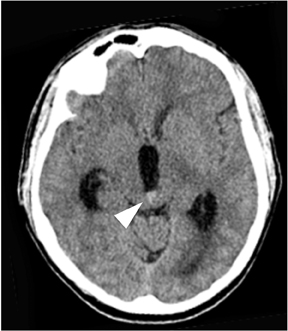
In the remaining 20% of cases, a nonaneurysmal cause is responsible. Roughly half of such cases are due to nonaneurysmal perimesencephalic hemorrhage, which is thought to result from venous bleeding.10,12 In these patients, the hemorrhage is located in the interpeduncular cistern and immediately anterior to the brainstem (Figure 3).13 Recognizing this pattern is important, as it helps to determine prognosis and to guide subsequent imaging.
Patients with isolated perimesencephalic hemorrhage almost always have no evidence of aneurysm on angiography, fare much better clinically than patients with aneurysmal hemorrhage, and are not at risk for recurrent hemorrhage.14 Other causes of angiogram-negative SAH include trauma, drug abuse (especially cocaine abuse), sickle cell disease, and coagulopathy.15
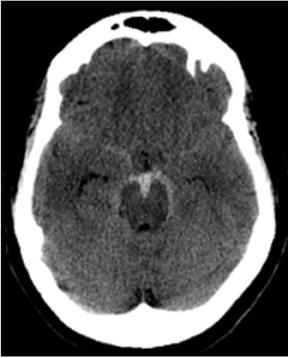
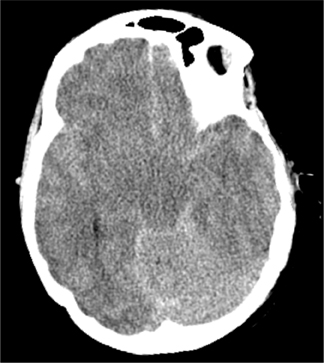
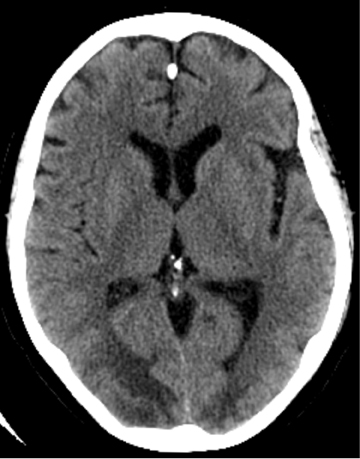
When adequate clinical information is available, CT diagnosis of SAH does not suffer from high rates of false-positive interpretations.Nevertheless, several mimics of SAH can manifest on CT as increased density in the subarachnoid space. Pseudo-subarachnoid hemorrhage is one such mimic; it can occur in the setting of markedly increased intracranial pressure, such as diffuse cerebral edema (Figure 4). The increased density in the subarachnoid space in pseudo-subarachnoid hemorrhage has been postulated to be due to engorgement of pial vasculature combined with heightened vascular conspicuity due to decreased parenchymal attenuation.16
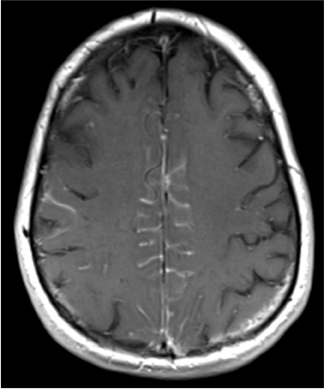
Leptomeningeal spread of tumor may cause increased attenuation of the subarachnoid space on CT, particularly in neoplasms with highnuclear-to-cytoplasmic ratios, such as lymphoma (Figure 5). Finally, myelographic contrast material can mimic subarachnoid blood. Although one might expect a history of recent myelogram to be readily available from patients presenting with headache, the authors’ experience is that such details occasionally may not be immediately available when the myelogram has been performed at another medical facility.
Parenchymal hemorrhage
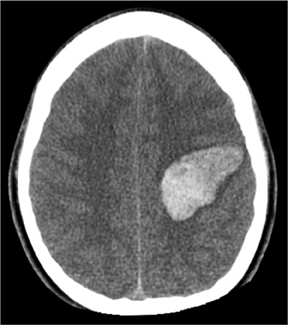
Many entities can cause brain parenchymal hemorrhage. An in-depth discussion of the pathophysiology and imaging of brain hemorrhageis beyond the scope of this article; a number of excellent review articles are available to explore this topic further.17,18 The major question faced by clinicians and radiologists is whether an underlying brain lesion, commonly vascular in nature, exists as a cause of hemorrhage. The answer to this question can often be quickly provided noninvasively by CTA or MRA (Figure 6). Clinicians can best select patients for angiographicimaging by considering several demographic, historical, and anatomical factors, most notably age, blood pressure, and hemorrhage location. In one study, CTA defined a vascular cause in 15% of unselected patients presenting with parenchymal hemorrhage.19 The incidence of a vascular cause of spontaneous parenchymal hemorrhage in this study rose to 47%, however, among patients < 46 years old.19 In addition to patients under the age of 50, other factors associated with a vascular etiology include absence of hypertension, presence of subarachnoid or intraventricular hemorrhage, and hemorrhage location in either the temporal or frontal lobes.19
Hydrocephalus
Hydrocephalus should be considered as a potential etiology in patients presenting with severe headache. In some cases, especially when acute, untreated hydrocephalus can be fatal. Therefore, evaluation of any brain imaging study must focus on the caliber of the ventricularsystem. If prior imaging studies are available, clinicians should carefully compare ventricular for interval changes that might signal new onset of hydrocephalus.
Deciding if the ventricular size is abnormally increased in a given patient often requires the subjective judgment of the radiologist. In earl yhydrocephalus, or in patients with brain volume loss due to aging or parenchymal disease, correctly identifying hydrocephalus may be difficult.Focusing on one particular location, the temporal horn of the lateral ventricle may prove helpful. Disproportionate enlargement of the temporal horns often indicates hydrocephalus, and can be useful in distinguishing ex vacuo dilation of the ventricles (ie, dilation due to parenchymal volume loss) from true hydrocephalus.20
Once hydrocephalus is detected, the next step is to determine whether the hydrocephalus is communicating or noncommunicating. Noncommunicating hydrocephalus results from a lesion in the ventricular system that obstructs flow of CSF. Its presence is suggested by the coexistence of a dilated proximal ventricular system and a decompressed distal ventricular system. The point of transition between the dilated and decompressed ventricles should be carefully scrutinized for the presence of a mass. Because the anatomic “choke points” of the ventricular system are located near the midline, one must be careful to scrutinize midline structures, including the foramen of Monro, the aqueduct of Sylvius, and the inferior fourth ventricle (Figure 7). Communicating hydrocephalus, by contrast, shows dilation of the entire ventricular system. In these cases,current or prior diseases affecting the CSF, such as subarachnoid hemorrhage, meningitis, and CSF dissemination of tumor, should be investigated.
Ischemia
Arterial infarction is relatively often accompanied by headache, particularly in young patients or those with history of migraine.21 Detecting arterial infarction on NCT depends on the duration and severity of vascular occlusion. In general, most patients show ischemic changes on NCT within 6 hours of symptom onset.22 Once arterial ischemia is recognized, the etiology should be sought to help gauge the risk of recurrence and determine optimal treatment.23 Fortunately, the neurologic deficits associated with arterial ischemia generally help distinguish these patients from patients with benign headaches.24
PRES
Posterior reversible encephalopathy syndrome (PRES) is a neurological syndrome that manifests on imaging studies as multifocal areas of edema usually involving the parieto-occipital white matter, but often also involving other areas, including cortical and subcortical watershed distributions, and occasionally the cerebellum, basal ganglia, or brainstem (Figure 8).25 Headache is often a clinical feature of PRES, although usually not the only presenting feature. Typically, patients with PRES will also exhibit seizures, visual disturbances, and alteration of consciousness.26 Furthermore, PRES is most commonly observed in conjunction with particular disease states, especially hypertension, eclampsia/preeclampsia, immunosuppression, chemotherapy, and autoimmune diseases.27 The combination of suggestive imaging patterns, typical clinical presentation, and predisposing conditions should suggest the diagnosis.
Although PRES abnormalities are best seen and characterized on MRI, they are usually visible on CT. One study compared CT and MR detection of PRES, and found that CT showed abnormalities in most cases of PRES (78%), but that MRI provided diagnosis with greater specificity.28 In that study, CT provided a specific diagnosis in only 45% of cases. In situations where the clinical findings are suggestive but initial CT is negative or equivocal, MRI should be performed for confirmation.
Brain tumor
Headache is common in patients with brain tumors.29 As in the case of PRES, however, headache usually is not the only presenting clinical feature in patients with newly diagnosed intracranial tumors; patients usually have coexistent neurologic deficits. In one study of 183 patients presenting with a brain tumor, isolated headache was the clinical presentation in only 8% of patients.30 Because primary headaches are much more common than tumors as a cause of headache, brain tumors overall are not a common cause of acute headache, with an incidence of < 1% among patients undergoing imaging for headache.31 Although full characterization may require further imaging, brain tumors of sufficient size to cause headache are often readily visible on NCT.
Conclusion
Patients presenting with severe headaches can present a diagnostic challenge due to the wide variety of causes that can range from benign to self-limiting to life threatening. Noncontrast CT plays a major role in the initial work-up of these patients. Therefore, awareness of the diseases that commonly show abnormalities on the initial CT is critical to development of an active search pattern.
References
- Goldstein JN, Camargo CA, Jr., Pelletier AJ, Edlow JA. Headache in United States emergency departments: Demographics, work-up and frequency of pathological diagnoses. Cephalalgia. 2006;26:684-690.
- Detsky ME, McDonald DR, Baerlocher MO, et al. Does this patient with headache have a migraine or need neuroimaging? JAMA. 2006;296:1274-1283.
- Morgenstern LB, Huber JC, Luna-Gonzales H, et al. Headache in the emergency department. Headache. 2001;41:537-541.
- Perry JJ, Stiell IG, Sivilotti ML, et al. High risk clinical characteristics for subarachnoid haemorrhage in patients with acute headache: Prospective cohort study. BMJ.2010;341:c5204.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000;342:29-36.
- Al-Shahi R, White PM, Davenport RJ, Lindsay KW. Subarachnoid haemorrhage. BMJ. 2006;333:235-240.
- Vermeulen MJ, Schull MJ. Missed diagnosis of subarachnoid hemorrhage in the emergency department. Stroke. 2007;38:1216-1222.
- Carley S, Wallmann P. Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Does a normal CT scan rule out a subarachnoid haemorrhage? Emerg Med J. 2001;18:271-273.
- Van der Wee N, Rinkel GJ, Hasan D, van Gijn J. Detection of subarachnoid haemorrhage on early CT: Is lumbar puncture still needed after a negative scan? J Neurol Neurosurg Psychiatry. 1995;58:357-359.
- Vermeulen M, van Gijn J. The diagnosis of subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 1990;53:365-372.
- Scotti G, Ethier R, Melancon D, et al. Computed tomography in the evaluation of intracranial aneurysms and subarachnoid hemorrhage. Radiology. 1977;123:85-90.
- Van der Schaaf IC, Velthuis BK, Gouw A, Rinkel GJ. Venous drainage in perimesencephalic hemorrhage. Stroke. 2004;35:1614-1618.
- Rinkel GJ, Wijdicks EF, Vermeulen M, et al. Nonaneurysmal perimesencephalic subarachnoid hemorrhage: CT and MR patterns that differ from aneurysmal rupture. AJNR Am J Neuroradiol. 1991;12:829-834.
- Greebe P, Rinkel GJ. Life expectancy after perimesencephalic subarachnoid hemorrhage. Stroke. 2007;38:1222-1224.
- Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm. A review of the causes. Stroke. 1993;24:1403-1409.
- Given CA, 2nd, Burdette JH, Elster AD, Williams DW 3rd. Pseudo-subarachnoid hemorrhage: a potential imaging pitfall associated with diffuse cerebral edema. AJNR Am J Neuroradiol. 2003;24:254-256.
- Fischbein NJ, Wijman CA. Nontraumatic intracranial hemorrhage. Neuroimaging Clin N Am. 2010;20:469-492.
- Dainer HM, Smirniotopoulos JG. Neuroimaging of hemorrhage and vascular malformations. Semin Neurol. 2008;28:533-547.
- Delgado Almandoz JE, Schaefer PW, Forero NP, et al. Diagnostic accuracy and yield of multidetector CT angiography in the evaluation of spontaneous intraparenchymalcerebral hemorrhage. AJNR Am J Neuroradiol. 2009;30:1213-1221.
- LeMay M, Hochberg FH. Ventricular differences between hydrostatic hydrocephalus and hydrocephalus ex vacuo by computed tomography. Neuroradiology. 1979;17:191-195.
- Tentschert S, Wimmer R, Greisenegger S, et al. Headache at stroke onset in 2196 patients with ischemic stroke or transient ischemic attack. Stroke. 2005;36:e1-3.
- Tomura N, Uemura K, Inugami A, et al. Early CT finding in cerebral infarction: Obscuration of the lentiform nucleus. Radiology. 1988;168:463-467.
- Rovira A, Grive E, Alvarez-Sabin J. Distribution territories and causative mechanisms of ischemic stroke. Eur Radiol. 2005;15:416-426.
- Provenzale JM. Imaging evaluation of the patient with worst headache of life--it’s not all subarachnoid hemorrhage. Emerg Radiol. 2010;17: 403-412.
- McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189:904-912.
- Fugate JE, Claassen DO, Cloft HJ, et al. Posterior reversible encephalopathy syndrome: Associated clinical and radiologic findings. Mayo Clin Proc. 2010;85:427-432.
- Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29:1036-1042.
- Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28:1320-1327.
- Loghin M, Levin VA. Headache related to brain tumors. Curr Treat Options Neurol. 2006;8:21-32.
- Vazquez-Barquero A, Ibanez FJ, Herrera S, et al. Isolated headache as the presenting clinical manifestation of intracranial tumors: A prospective study. Cephalalgia. 1994;14:270-272.
- Evans RW. Diagnostic testing for the evaluation of headaches. Neurol Clin. 1996;14:1-26.
Related Articles
Citation
Imaging “worst headache of my life” Part 1: Conditions in which the initial CT is often positive. Appl Radiol.
July 4, 2013