Imaging spinal stenosis
Images




Degenerative lumbar spinal stenosis is a condition in which there is diminished space available for the neural and vascular elements in the lumbar spine secondary to degenerative changes in the spinal canal.1 Classically, patients with spinal stenosis complain of lower-extremity pain exacerbated by walking and relieved by bending forward or sitting. Given that spinal stenosis is the most common reason for lumbar spine surgery in patients over 65 years,2 and that many patients with anatomic narrowing are asymptomatic,3 there is a recognized need for standardizing descriptive radiologic terms for spinal stenosis. The variability in description and reporting of spinal stenosis among radiologists and other physicians is well-documented.4 This variability and lack of standardization may contribute to increased heterogeneity of the patient population undergoing surgery for spinal stenosis, rendering any analysis of surgical outcomes difficult at best. In response, a combined task force of radiologists and orthopedic surgeons endorsed a set of radiologic criteria for spinal stenosis in hope of improving communication among healthcare providers. Their recommendations for lumbar disc nomenclature were released in 2001 and revised in 2014.5,6
The diagnosis of spinal stenosis relies primarily on imaging to provide objective evidence of neurovascular compromise. The imaging features may be roughly classified into two categories; qualitative and quantitative findings. In 2011, Steurer and associates conducted a review of quantitative radiologic criteria published in the literature, and compiled a list of descriptive terms for lumbar spinal stenosis.7 In 2012, Mamisch and associates surveyed an expert panel to learn which imaging criteria were considered most important for the diagnosis of spinal stenosis, and to assess the strength of agreement among experts. At the end of their survey, Mamisch et al concluded that while some qualitative criteria were considered important by imaging experts, there were no widely accepted quantitative criteria for the diagnosis of spinal stenosis.8
In this article, we will discuss pertinent anatomy, updated nomenclature, indications for imaging, and qualitative and quantitative criteria, illustrating our discussion of stenonis with examples for clarity where appropriate.
Anatomy, nomenclature, and the combined task force
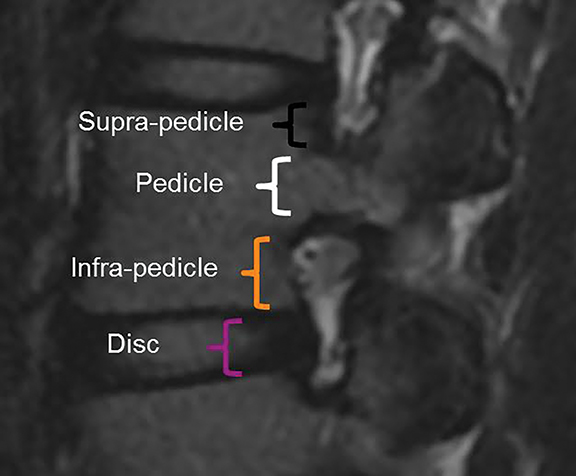
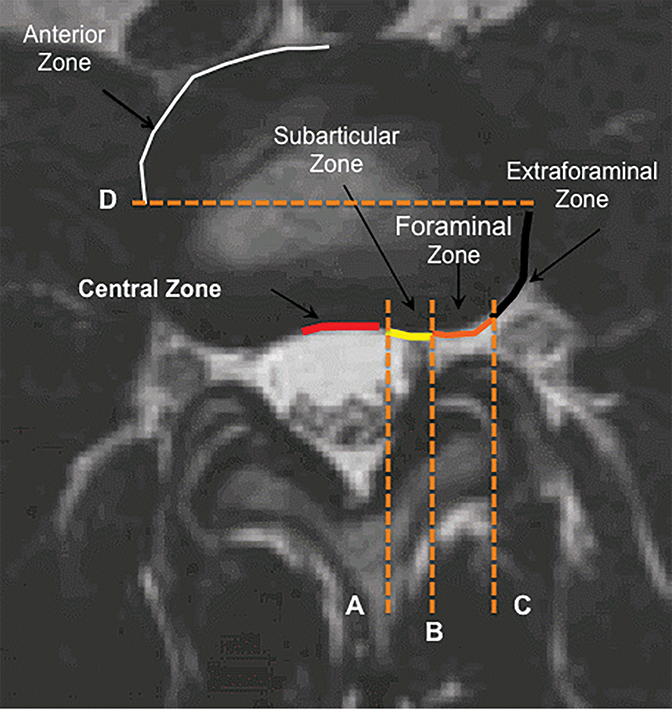
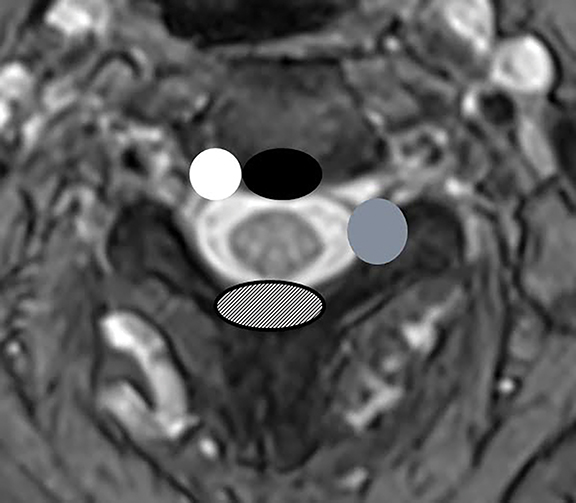
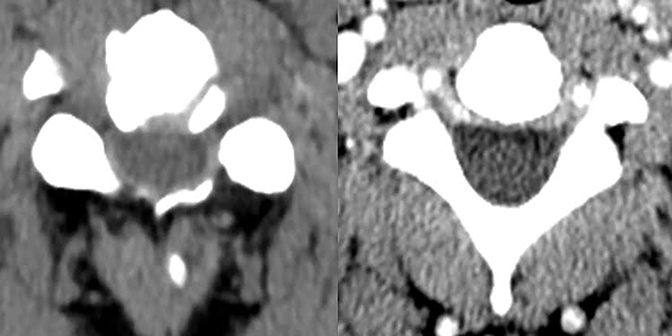
The purpose of the most recent version of the recommendations of combined task force comprised of North American Spine Society (NASS), American Society for Spine Radiology (ASSR), and the American Society of Neuroradiology (ASNR) is to improve communication between radiologists and clinicians. The nomenclature clearly defines anatomy in terms of zones and levels in order to standardize localization of pathology. In the sagittal plane, the pedicle serves as a boundary to describe levels with the different levels being disc level, suprapedicle level, pedicle level and infrapedicle level (Figures 1 and 2). Zones (central, subarticular, foraminal, extraforaminal and anterior zones) are defined in the axial plane and are illustrated and described in Figure 3.
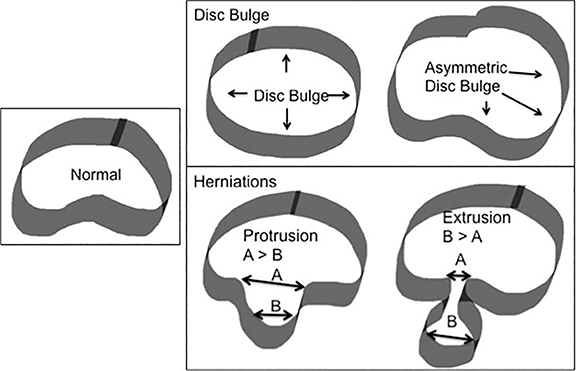
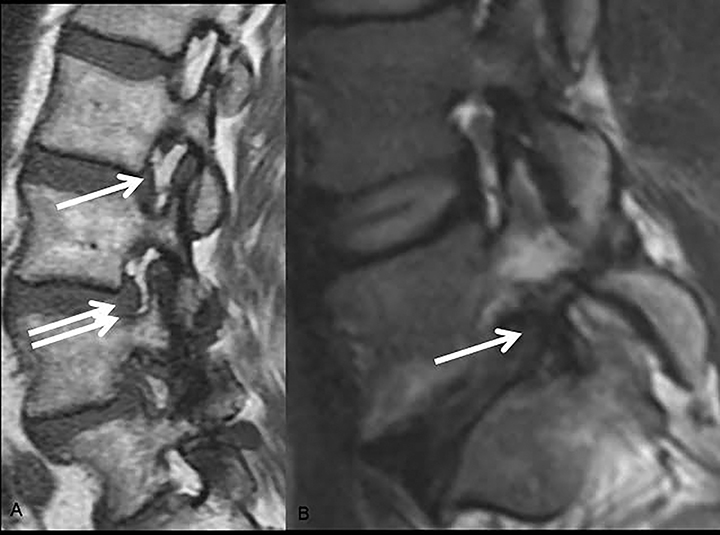
The nomenclature also defines the differences between disc herniation and disc bulge (Figure 4). Disc herniation is a broad term that encompasses the various manifestations of degenerative disc disease with extension of disc material beyond the edges of the vertebral body endplates; contrary to disc bulge, herniation is a focal extension involving less than 25% of disc circumference. Disc bulge is more diffuse (involving more than 25% of disc circumference) and represents mild (3mm or less), extension of the disc material beyond the disc space; bulge may be symmetric or asymmetric.
Disc herniation may be further classified into protrusion or extrusion. In protrusion, the greatest diameter of the herniated disc is less than its base at the site of herniation from the parent disc; extrusion describes a herniated disc, which has a maximum diameter greater than its base. A further sub-classification of disc extrusion is a sequestrated disc. Sequestrated discs are herniated discs that have no visible connection to the parent disc on any imaging plane. An overlooked or misdiagnosed sequestrated disc that migrates away from the parent disc level is a known cause of failed back surgery.9
It is important to carefully evaluate both, the zones (central, subarticular, foraminal, and extraforaminal) and levels (disc, supra-pedicular, pedicular, infra-pedicular) when assessing degenerative disease. Disc herniations, bony hypertrophy, and ligamentous changes can compromise the spinal canal and cause stenosis in predictable locations (Figure 5). Foraminal stenosis can occur in the anteroposterior or craniocaudal direction as a result of disc herniation, facet hypertrophy or anteroposterior subluxation; the latter resulting from craniocaudal subluxation (Figure 6). In the lumbar spine, the inferior portion of the neural foramen narrows initially. Narrowing of the nerve-bearing, upper portion of the foramen is a late finding.
Indications for imaging stenosis
Typical clinical features of stenosis include buttock or lower extremity pain with a positive straight leg raise test, radiculopathy or neurogenic claudication. Neurogenic claudication has been variably defined by radiculopathy or pain in the lower extremity that worsens with walking and improves with sitting or bending forward.1,10 The presence of these clinical findings suggests disc herniation and/or stenosis. Uncomplicated acute low back pain or radiculopathy is a benign, self-limited condition that does not warrant any imaging studies. Imaging is considered in those patients who have had 4 to 6 weeks of medical management and physical therapy that resulted in little or no improvement in their back pain. Imaging may be considered earlier if there is a history of malignancy, concern for infection, a fracture, symptoms of true myelopathy (progressive or severe neurologic deficits), in the setting of cauda equina syndrome (urinary retention, fecal incontinence, motor deficit at multiple levels, and saddle anesthesia), or with history of back surgery.11,12,13 Degenerative changes are more common with increasing age. As these findings may be seen in both symptomatic and asymptomatic individuals, it is important that imaging findings be correlated with the physical exam.
Imaging modalities in stenosis
Radiographs
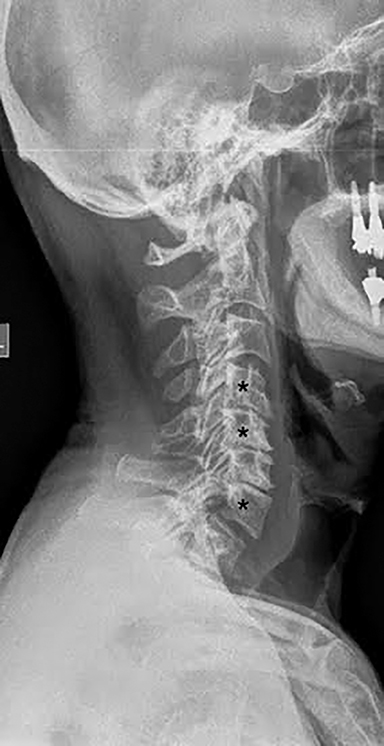
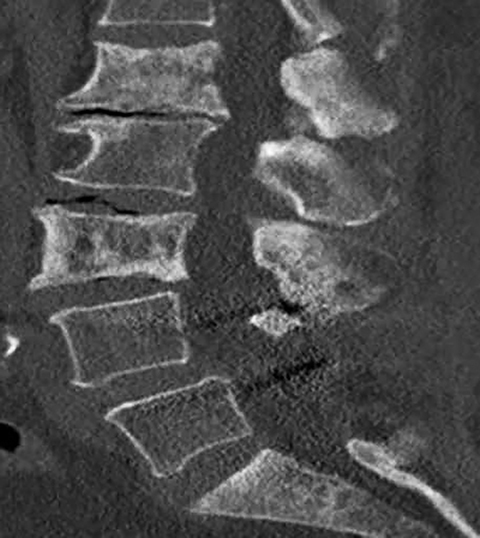
Radiographs are often the initial means of evaluating back pain. Radiographs are low cost, readily available, and can assess for degenerative changes of disc height loss, vacuum phenomenon, osteophytes, and vertebral alignment (Figure 7). Unrelated causes of back pain, such as sacroiliac joint pathology, renal stones, or calcified aneurysmal dilatation of the aorta may also be identified. Soft tissue, disc and nerve evaluation are limited, and radiographs are insensitive for metastases and infection.14
Computed tomography
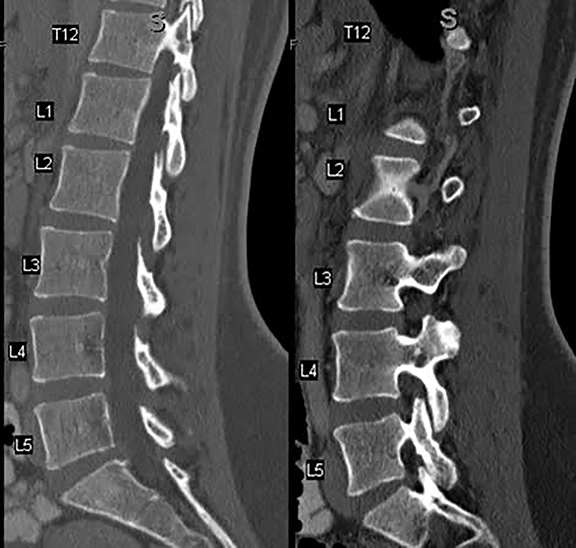
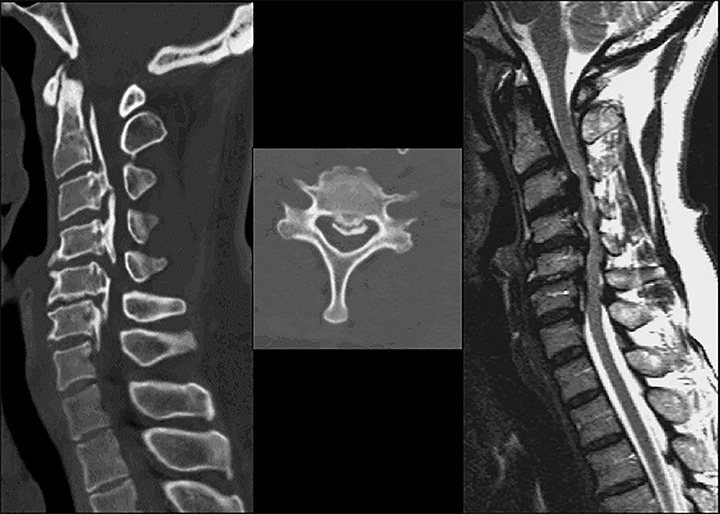
Computed tomography is the best modality to depict bony anatomy for presurgical planning. It can also diagnose disc herniation and spinal stenosis and is superior to radiographs in detecting metastases and infection (Figures 8 and 9).1,14 Nerve-root impingement is not reliably detected and has the added disadvantage of radiation exposure. Although typically performed without contrast, contrast-enhanced CT has been shown to provide improved visualization of disc pathology by evaluating for mass effect on the epidural venous plexus. Epidural enhancement surrounding a herniated disc can assist in its detection (Figure 10).15
For patients unable to have an MRI or who have had an inconclusive MRI, CT myelogram can serve as an alternative. Although this is an invasive procedure, contrast in the subarachnoid space outlines the neural structures and is comparable to MRI in detecting stenosis and neural impingement (Figure 11).1 CT myelogram is also useful in diagnosing CSF leak and nerve root avulsion.
Magnetic resonance imaging
Magnetic resonance imaging is the modality of choice to evaluate stenosis and disc pathology.1,14 MRI has many advantages: it is noninvasive, has no ionizing radiation, has high sensitivity in diagnosing stenosis, has high soft tissue contrast, and it best depicts cord, nerve roots, and bone marrow abnormalities.1,14 Standard MRI sequences in the lumbar spine may include sagittal T1-weighted, T2-weighted, STIR, and proton density-weighted, and axial T1- and T2-weighted sequences (Figures 12 and 13). In addition, contrast enhanced MRI may be necessary for indications such as infection, tumor, and post surgical evaluation. Note that T2-weighted GRE sequence, often used in cervical spine imaging, may overestimate stenosis and should be correlated with other sequences. MR images can also be degraded by susceptibility artifact from metallic hardware and may be contraindicated in some patients. In patients with history and physical examination findings consistent with degenerative lumbar spinal stenosis, MRI is suggested as the most appropriate, noninvasive test to confirm the presence of anatomic narrowing of the spinal canal or nerve root impingement.1
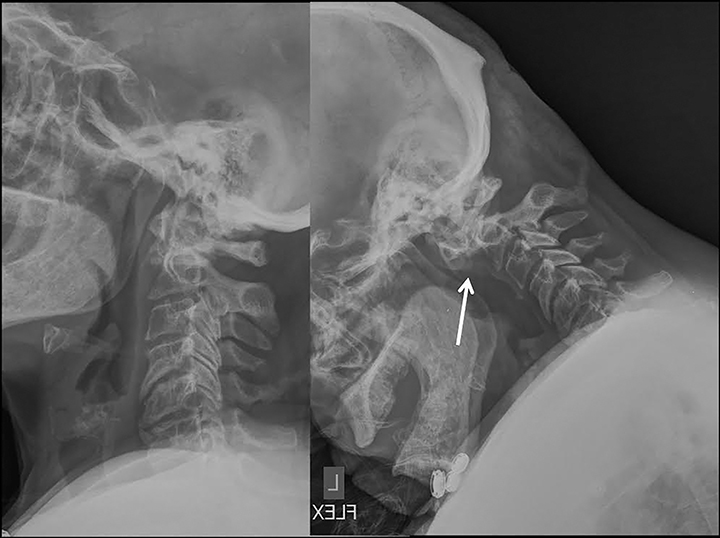
Dynamic flexion-extension radiographs (Figure 14) and CT and MRI with load bearing may also be performed as a useful adjunct.1
Qualitative diagnostic criteria
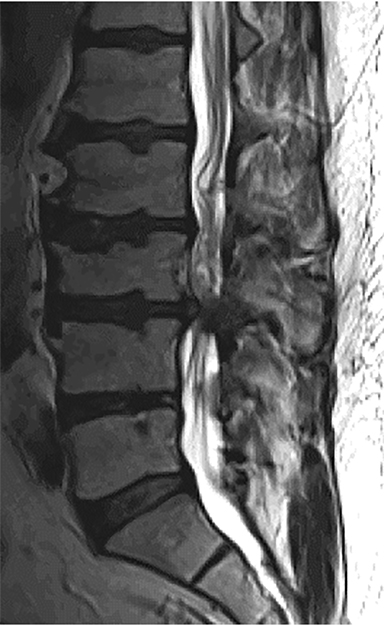
The qualitative imaging findings in stenosis of the lumbar spine may be broadly classified into the specific causes of anatomic narrowing and their effect on neural elements. While this discussion of stenosis centers on the lumbar spine, the principles may also be applied to the cervical and thoracic regions. The main causes of spinal canal and neuroforaminal narrowing in the lumbar spine include disc herniation, facet hypertrophy, and ligamentum flavum hypertrophy/infolding. Additional causes of stenosis related to degenerative disease include synovial cysts, and ossification of the posterior longitudinal ligament.
Common causes of anatomic narrowing
Disc herniation
As previously discussed, disc herniations are an important cause or contributor to stenosis (Figure 15). Several interventional therapies focus on disc removal, and accurate, reproducible radiologic description is imperative for optimal surgical outcomes.
Facet and ligamentum flavum hypertrophy
Facet and ligamentum flavum hypertrophy frequently co-exist. Facet hypertrophy refers to bony overgrowth at the facet joints of the lumbar spine on a degenerative basis. The bony overgrowth may then result in narrowing of the lateral recess or neural foramen. Spinal canal compromise may also occur, when superimposed disc herniation and ligamentum flavum hypertrophy are present.
Ligamentum flavum hypertrophy or infolding refers to abnormal thickening and buckling of the ligamentum flavum as a result of degenerative changes in the lumbar spine. It is frequently bilateral and causes posterior obliteration of the CSF space in the thecal sac.
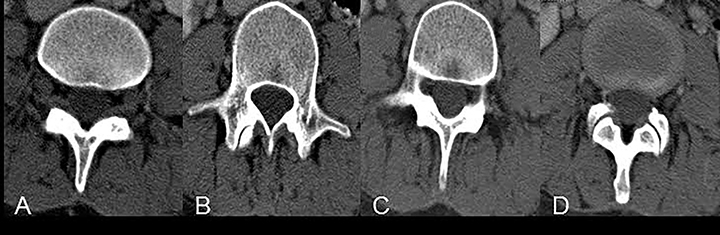
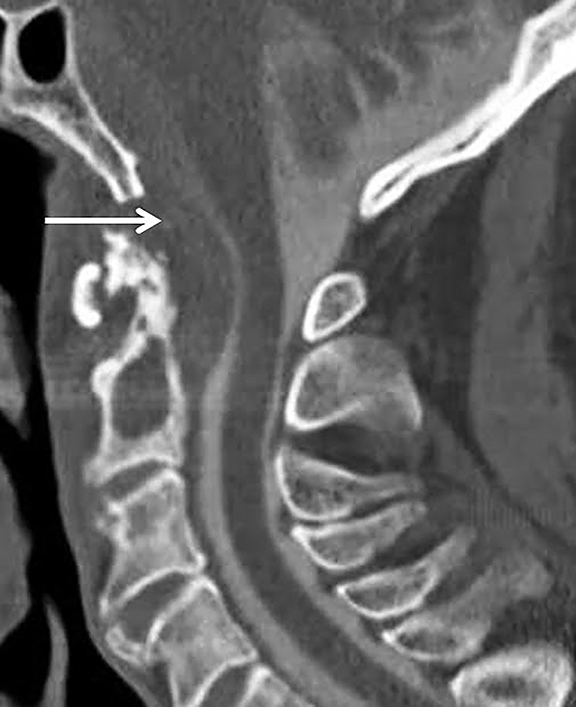
One important cause of cervical spinal canal stenosis that bears mention is ossification of the posterior longitudinal ligament (OPLL).16 This diagnosis commonly co-exists with diffuse idiopathic skeletal hyperostosis (though not always), and is most easily appreciated on CT (Figure 16). In the cervical spine, OPLL may result in spinal injury after minor cervical trauma due to pre-existing cervical canal stenosis.
Effect on neural elements
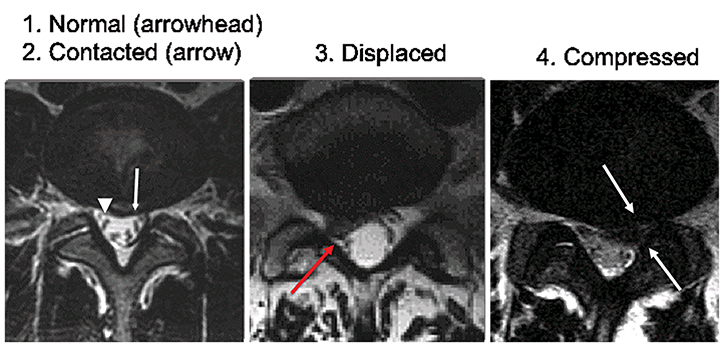
The effect of spinal canal stenosis may be inferred by directly visualizing the neural structures on high-quality MR images. In the spinal canal, the effect on the spinal cord may be reported descriptively by noting whether the CSF space is merely obliterated, or whether there is deformity of the spinal cord. The presence or absence of abnormal signal in the spinal cord should also be noted. The nerve roots of the cauda equina may also show crowding and redundancy above the site of narrowing (Figure 17). Pfirrmann et al proposed a grading system for disk herniation-related nerve root compromise that showed high correlation with intraoperative findings during subsequent lumbar spine surgery.17 In the Pfirrmann grading scheme, nerve root compromise is classified into four grades; normal, contact without displacement or compression, displacement, and compression, as shown in Figure 18. Grading of neural foraminal stenosis may be graded by noting effacement of the fat surrounding the nerve roots or by direct observation of nerve root displacement or compression with morphologic change.
Quantitative diagnostic criteria
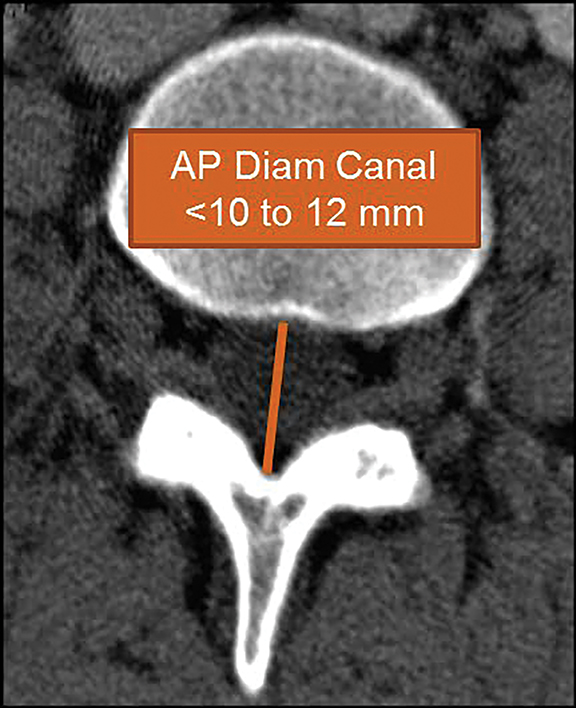
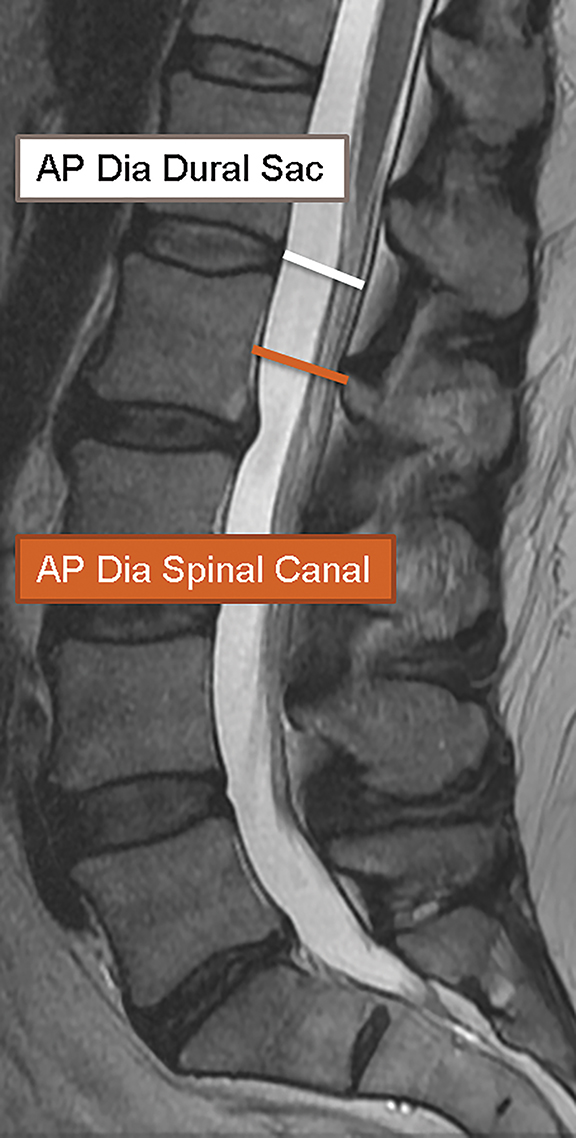
In 2011, Steurer and associates conducted a review of quantitative radiologic criteria published in the literature, and compiled a list of descriptive terms for lumbar stenosis.7 The quantitative diagnostic criteria for spinal canal stenosis are less widely accepted,8 and only the mostrelevant are discussed in this article. For the evaluation of the spinal canal, stenosis is compatible with an AP diameter of the canal less than 10 mm in the cervical spine or 12 mm in the lumbar spine (Figure 19). On MR images, a mid-sagittal diameter of the dural sac less than 10 mm is also consistent with stenosis (Figure 19). Separate evaluation of the dural sac diameter is useful in cases where there is normal bony canal diameter on non-contrast CT images, for example epidural lipomatosis causes mass effect on the dural sac with normal spinal canal diameters. Additional imaging criteria such as cross-sectional area of the dural sac and transverse diameter of the osseous spinal canal are also published in the literature.7
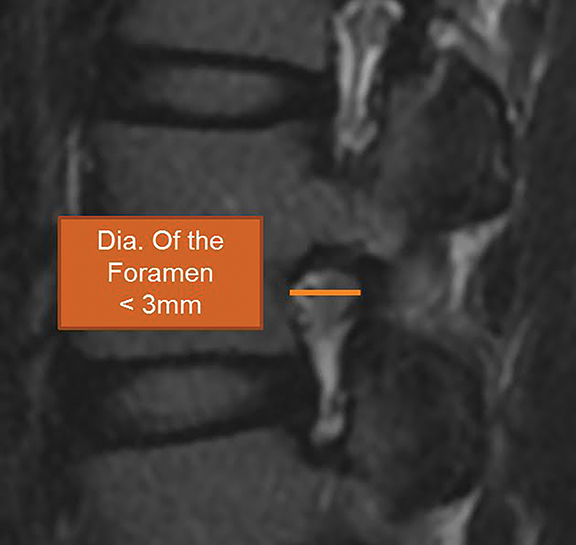
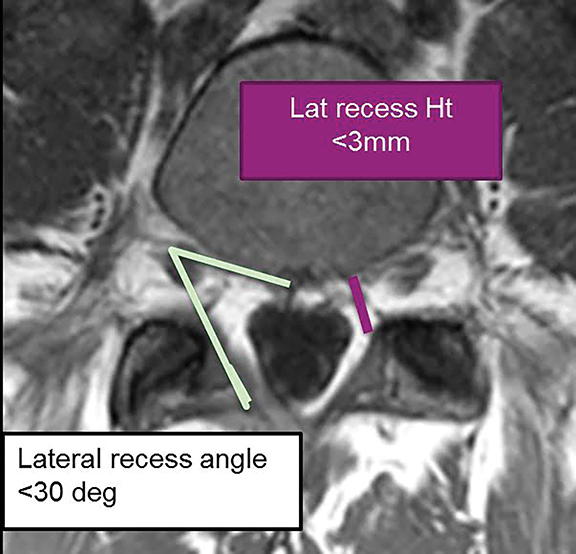
For evaluation of the neuroforamen, an anteroposterior diameter of the foramen of less than 3mm on sagittal images is considered diagnostic for stenosis (Figure 20A). A lateral recess height less than 3mm or lateral recess angle less than 30 degrees is also further evidence of spinal stenosis (Figure 20B). 7
The joint forces in the revised lumbar spine nomenclature recommendations6 suggest that spinal canal stenosis can be graded as mild, moderate, or severe if the canal is narrowed by less than a third, one-third to two-thirds, or greater than two-thirds of the original diameter, respectively. A similar grading system can be employed for the neural foramen.
Conclusions
The imaging evaluation of spinal stenosis continues to evolve, with a move towards standardization and validation of diagnostic criteria. The combined task forces of the NASS, ASSR and ASNR is a primary driver of this movement towards standardization of lumbar stenosis nomenclature, and they continue to advocate a simple, reproducible, easily understood scheme for the evaluation of spinal stenosis. While the inherent value of accurate, standardized reporting of spinal stenosis is well recognized, it is important to remember that clinical significance depends on correlation with clinical data and cannot be inferred from morphologic data alone.
References
- Kreiner DS, Shaffer WO, Baisden JL, et al. An evidence-based clinical guideline for diagnosis and treatment of degenerative lumbar spinal stenosis. Spine J. 2013; 13(7):734-743.
- Deyo R, Gary D, Keurter W, et al. United States trends in lumbar fusion surgery for degenerative conditions. Spine. 2005;30(12):1441-1445.
- Brinjiki W, Luetmer P, Comstock B, et al. Systematic literature review of imaging features of spinal degeneration in asymptomatic populations. AJNR American Journal of Neuroradiology. 2015;36(4):811-816.
- Brant-Zawadski M, Jensen M, Obuchowski N, et al. Imaging corner: spinal nomenclature. Inter- and intra-observer variability in interpretation of lumbar disc abnormalities: a comparison of two nomenclatures. Spine. 1995;20(11):388-390.
- Fardon DF, Milette PC. Nomenclature and classification of lumbar disc pathology: recommendations of the combined task forces of the North American Spine Society, American Society of Spine Radiology and American Society of Neuroradiology. Spine (Phila Pa 1976) 2001; 26: E93–113.
- Fardon D, Williams A, Dohring E, et al. Lumbar disc nomenclature: version 2.0 recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014;14(11):2525-2545
- Steurer J, Roner S, Gnannt R, et al. Quantitative radiologic criteria for the diagnosis of lumbar spinal stenosis: a systematic literature review. BMC Musculoskelet Disord. 2011;12(1):175-183.
- Mamisch N, Brumann M, Hodler J, et al. Radiologic criteria for the diagnosis of spinal stenosis: results of a Delphi survey. Radiology. 2012;264(1):174-179.
- Choi K, Lee J, Kim J, et al. Unsuccessful percutaneous endoscopic lumbar discectomy: a single-center experience of 10,228 cases. Neurosurgery. 2015;76(4):372-380.
- Genevay S, Atlas SJ, Katz JN. Variation in eligibility criteria from studies of radiculopathy due to a herniated disc and of neurogenic claudication due to lumbar spinal stenosis: a structured literature review. Spine. 2010; 35(7):803-811.
- Patel ND, Broderick DF, Burns J, et al. American College of Radiology ACR Appropriateness Criteria® Low Back Pain. Available at https://acsearch.acr.org/docs/69483/Narrative/ American College of Radiology. Accessed 12/12/2016.
- Chou R, Qaseem A, Owens DK, Shekelle P. Diagnostic imaging for low back pain: advice for high-value health care from the American College of Physicians. Ann Intern Med. 2011;154 (3):181–189.
- Arana E, Kovac F, Royuela A., et al. Influence of nomenclature in the interpretation of lumbar disk countour on MR imaging: a comparison of the agreement using the combined task force and the Nordic nomenclatures. AJNR Am J Neuroradiol. 2011;32(6):1143-1148.
- Modic M, Masaryk T, Ross J, et al. Imaging of degenerative disc disease. Radiology. 1988; 168(1):177–186.
- Russell D, D’Angelo C, Zimmerman R, et al. Cervical disc hernation: CT demonstration after contrast enhancement. Radiology. 1984; 152(3): 703-712.
- Shoichiro O, Michimasa M, et al. Ossification of posterior longitudinal ligament: MR evaluation. AJNR Am J Neuroradiol. 1992; 13:1059 – 1067.
- Pfirrmann C, Dora C, Schmid M, et al. MR image-based grading of lumbar nerve root compromise due to disk herniation: reliability study with surgical correlation. Radiology. 2004;230(2):583-588.
Citation
KS T, M C, E S, AE F. Imaging spinal stenosis. Appl Radiol. 2017; (1):8-17.
January 10, 2017