Imaging of thyroid nodules
Images





The worldwide incidence of thyroid cancer has increased over the past several decades,1 reaching an estimated incidence of 2.1% of all worldwide cancers in 2012.2 In the U.S., the incidence has tripled from 4.8 to 15 of 100,000 people between 1975 and 20143 and was estimated to be 3.3% of all cancers in the United States in 2012.2 This trend has been predominantly driven by a disproportionate increase in the diagnosis of small papillary thyroid cancers without a significant change in mortality (0.5 per 100,000 people).3 This has led many people to believe that the higher incidence is due to the detection of subclinical disease4-7 and possibly environmental factors.8
Diagnosis of subclinical disease in this situation has been termed over-diagnosis, defined as the detection of indolent thyroid cancer in asymptomatic patients or patients who will die from other causes. This increased detection of subclinical cancer may be harmful secondary to the psychological, physical, and financial burden associated with diagnostic testing and surgery.8 Fortunately, there has been a plateau in incidence over the past several years, suggesting stabilization rather than a continued upward trend.9
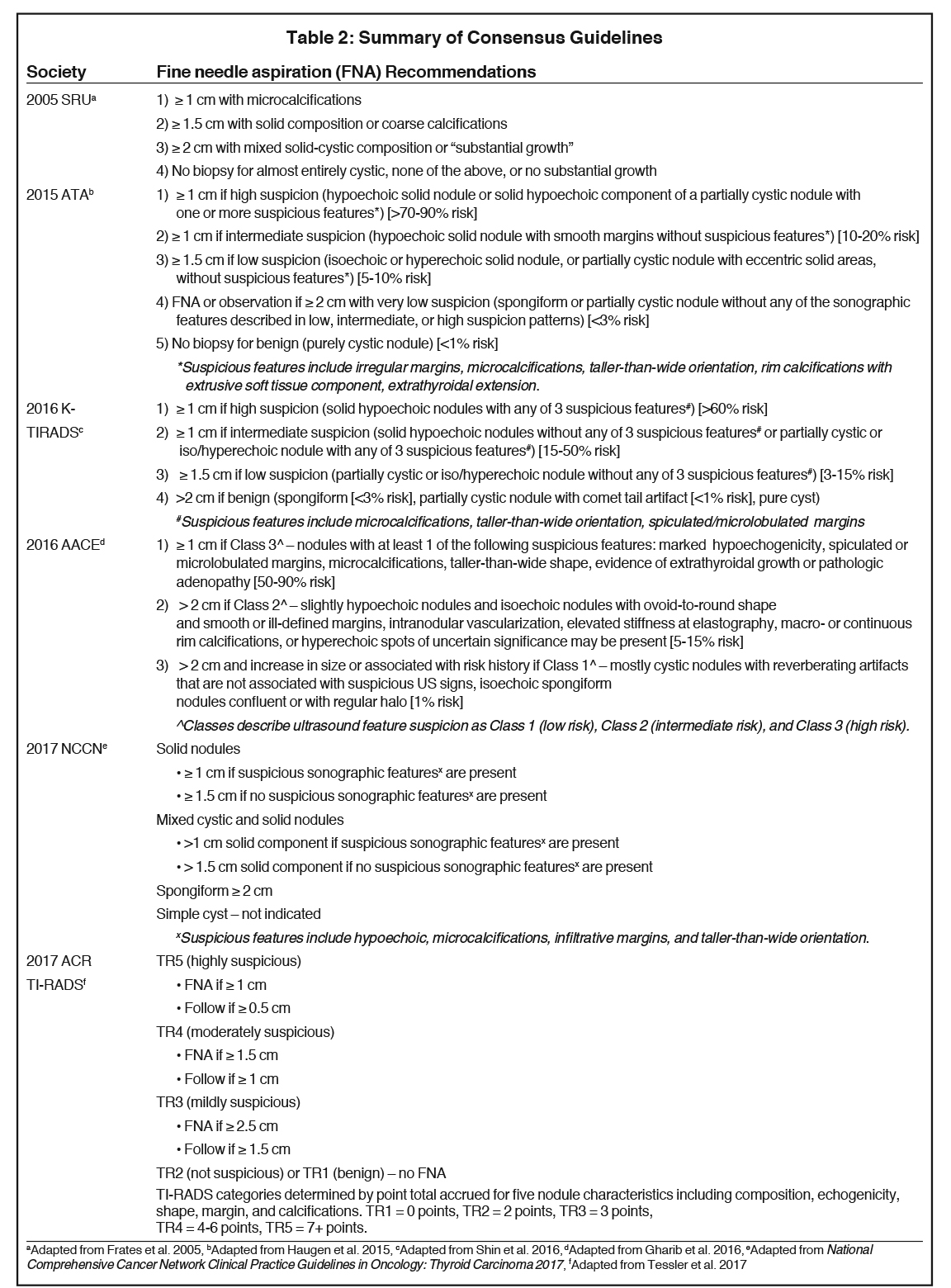
In this clinical context, the radiologist is faced with a difficult challenge -responsibly report clinically significant findings while balancing the fear of missing a cancer diagnosis. How can the radiologist differentiate benign and malignant thyroid nodules based on sonographic findings? The answer to that question determines recommendations for fine needle aspiration (FNA), surveillance, or nothing at all. Similarly, the radiologist is faced with the challenge of the incidental thyroid nodule (ITN), identified on computed tomography (CT), magnetic resonance imaging (MRI), or nuclear medicine studies, such as fluorodeoxyglucose-positron emission tomography (FDG-PET). When should the radiologist recommend dedicated thyroid ultrasonography for an ITN? Several multi-disciplinary professional societies have evaluated the available evidence and proposed guidelines to help the radiologist answer the above questions.
The purpose of this review is to provide the general radiologist with practical information regarding the management of thyroid nodules evaluated with ultrasonography while reviewing society guidelines. This review will also provide guidance on the management of ITNs detected on other imaging modalities (CT, MR, FDG-PET, and US) based on the American College of Radiology (ACR) Incidental Thyroid Findings Committee white paper.
Thyroid anatomy
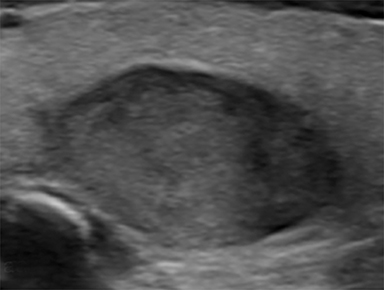
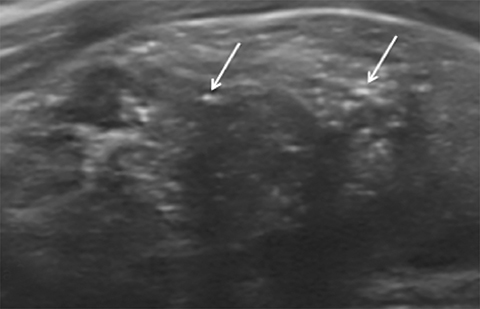
Located superficially in the infrahyoid neck, the normal thyroid gland (Figure 1) is composed of right and left lobes joined centrally at their inferior thirds by the isthmus, a thin band of thyroid parenchyma that crosses the midline anterior to the trachea. The thyroid is sandwiched between the strap and sternocleidomastoid musculature anteriorly and the longus colli musculature posteriorly. The common carotid arteries and internal jugular veins are located laterally.10
Thyroid imaging
Ultrasonography
Ultrasonography is the imaging modality of choice for evaluating thyroid nodules because of its widespread availability, low cost, and lack of ionizing radiation. In addition, the thyroid’s superficial location in the neck makes it accessible and amenable to high-frequency sonographic evaluation for accurate characterization. Lastly, visualization on ultrasonography is particularly useful for ultrasound-guided FNA. Multiple studies have reported a lower rate of non-diagnostic and false-negative cytology results from US-guided FNA compared to palpation-guided FNA.11,12
On ultrasonography, the normal thyroid gland is a well-circumscribed structure that is homogeneous in echotexture and hyperechoic relative to adjacent musculature. In the adult, each lobe measures 4-6 cm in length and up to 2 cm in width and thickness. The isthmus measures up to 3 mm in thickness.10
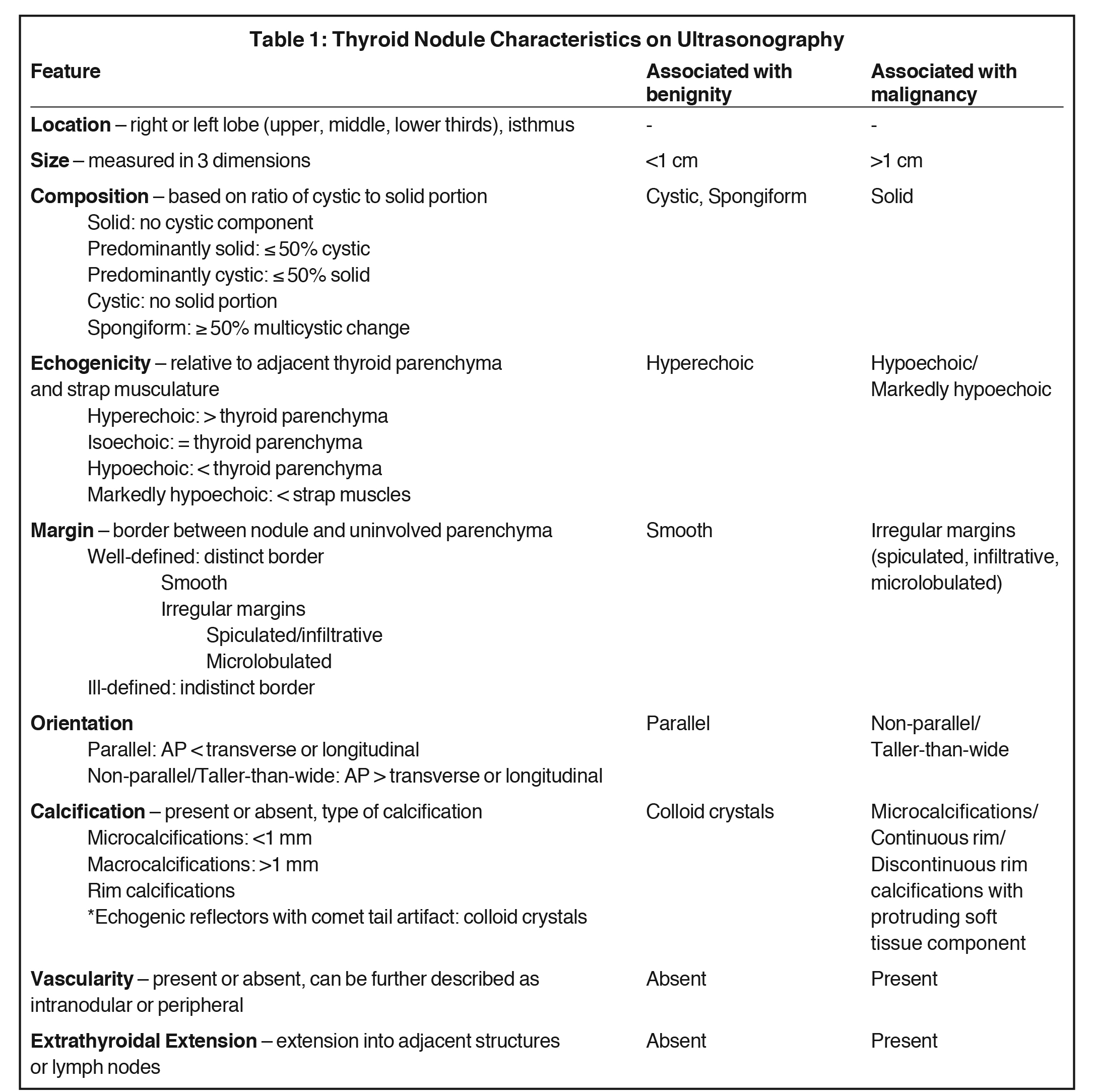
When evaluating a thyroid nodule, the location and size (in three dimensions) should be described. For nodules <0.5 cm, only the maximal diameter should be reported.13 Complete evaluation of a thyroid nodule should include sonographic features such as composition, echogenicity, margins, orientation, presence and type of calcifications, vascularity, and extrathyroidal extension, if present. The overall sonographic pattern in conjunction with size confers a malignancy risk and provides a basis for the radiologist to make a management recommendation.14,15 If there are multiple nodules, each nodule should be described and management decisions should be based on individual nodule suspicion, sometimes requiring multiple FNAs.16
Features associated with benignity include cystic or spongiform nodules as well as multiple nodules (without suspicious features) in an enlarged thyroid gland. Features associated with malignancy include hypoechogenicity, solid composition, irregular margins, taller-than-wide orientation and microcalcifications17 with the latter three having the highest specificities.16 The characteristics that should be included in the radiology report are described in more detail below and summarized in Table 1.
Multiple societies have created consensus statements to assist the radiologist and clinician in the management of thyroid nodules based on sonographic features, signifying the lack of a single generally accepted set of guidelines. These include the Society of Radiologists in Ultrasound,18 the American Thyroid Association (ATA),16 the American Association of Clinical Endocrinologists (AACE),19 the National Comprehensive Cancer Network,20 the ACR,21 and the Korean Society of Thyroid Radiology (KSThR).13 Several studies have compared and supported the validity of these guidelines.22-25 Table 2 summarizes the management guidelines for these groups.13,16,18-21
Lesion characteristics on ultrasonography
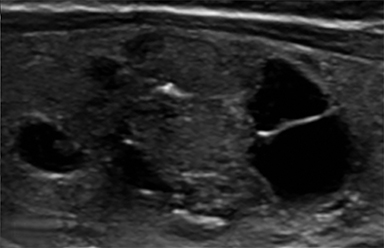
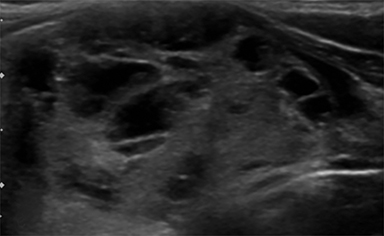
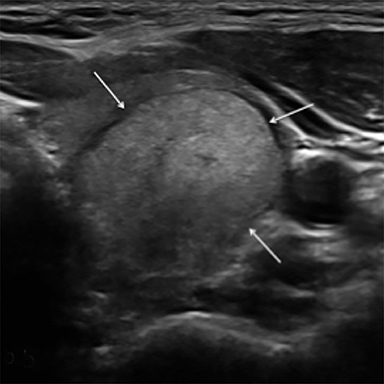
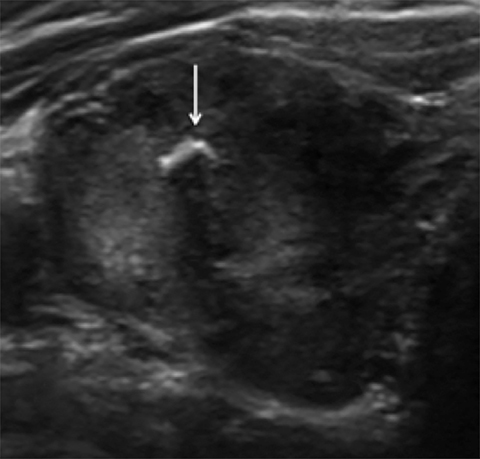
Composition is based on the ratio of cystic to solid components (Figure 2). Cystic lesions have no solid components, predominantly solid lesions have ≤50% cystic components, predominantly cystic lesions have <50% solid comnents, and solid lesions have no cystic components. Spongiform nodules have multiple microcysts in >50% of the nodule and are seen in benign colloid cysts (Figure 3).26
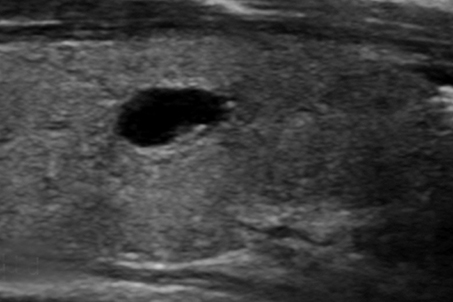
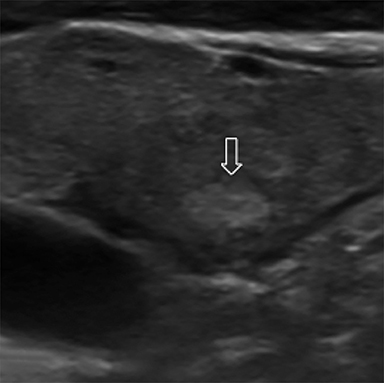
Nodule echogenicity (hypoechoic, isoechoic, hyperechoic) is described relative to thyroid parenchyma with hypoechogenicity having an association with malignancy (Figure 4).16 Markedly hypoechoic nodules are less echogenic than the adjacent strap muscles and been shown to have a higher malignancy risk (Figure 4D).13
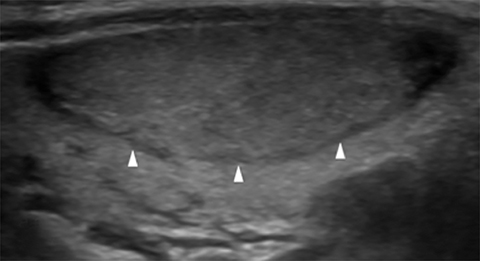
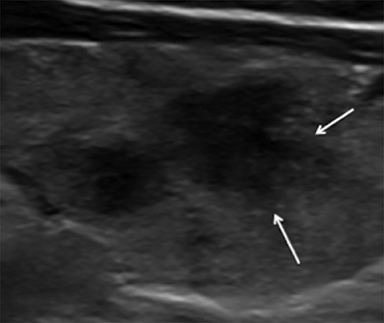
Nodule margins may be smooth, irregular (microlobulated, infiltrative/spiculated), and ill-defined (Figure 5). Nodules with smooth or irregular margins have a well-demarcated border between nodule and uninvolved parenchyma. Ill-defined nodules do not have a clear border and are nonspecific. Irregular margins (e.g. microlobulated, infiltrative/spiculated) are associated with malignancy.13,16,21
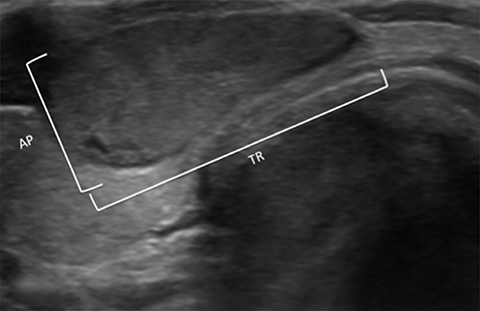
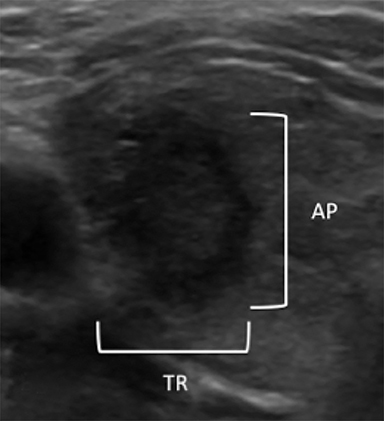
Orientation is defined as parallel (anteroposterior diameter is less than or equal to the transverse or longitudinal diameter) and non-parallel/taller-than-wide (anteroposterior diameter is greater than the transverse or longitudinal diameter) (Figure 6). A taller-than-wide orientation is less sensitive for malignancy although it is highly specific.13,16,26,27
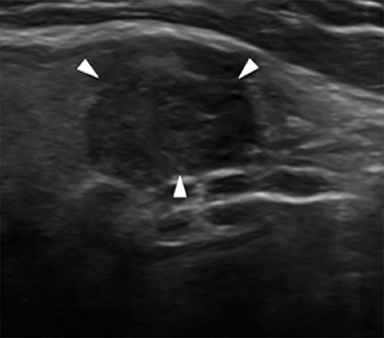
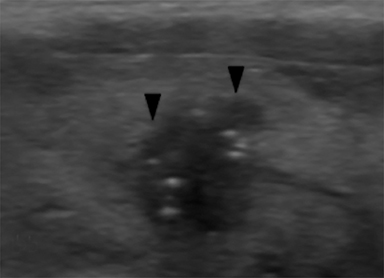
Microcalcifications are echogenic foci less than 1 mm and do not demonstrate acoustic shadowing (Figure 7). They are highly specific for papillary thyroid carcinoma particularly when associated with solid, hypoechoic nodules.13,21,28 Macrocalcifications (greater than 1 mm) are generally less worrisome, although discontinuous rim calcifications with a protruding soft-tissue component are concerning for malignancy.13,16 Of note, echogenic foci with comet tail artifact represent benign colloid crystals (Figure 3) and can easily be confused with microcalcifications.16,21
The presence of vascularity (intranodular or peripheral) may be suggestive of malignancy, but data regarding its reliability are mixed.13,16
Interval growth
Interval growth is defined as a minimum increase in total volume of 50%, correlating with a 20% increase in diameter (minimal increase of 2 mm in at least two dimensions).29 Although rapid growth of a nodule can be seen in high-grade malignancies such as anaplastic carcinoma and lymphoma, these are rare and typically show aggressive sonographic features. Multiple studies have shown that interval growth is not a reliable indicator of malignancy since both benign and malignant lesions can grow slowly or remain stable.30-33 As a result, the ATA recommends the decision for initial FNA or repeat FNA after indeterminate or benign cytology be based on sonographic characteristics rather than size increase.16
Extrathyroidal imaging
Multiple societies recommend a cervical lymph node evaluation in all patients who undergo thyroid ultrasonography with known or suspected thyroid nodules.16,21 Papillary thyroid carcinomas, which comprise 80% of all thyroid malignancies spread via the lymphatic system, as does medullary thyroid carcinoma.34 Nodes that should be evaluated include the cervical chain lymph nodes in both the lateral (levels II, III, IV, V) and central (level VI) compartments. Similar to thyroid nodules, sonographic features and morphology are most important in determining risk of malignancy. Suspicious sonographic features include round shape, loss of the fatty hilum, calcifications, cystic change, increased echogenicity, and increased vascularity.19,34 These sonographic features are more important in management than size, which is nonspecific. However, the radiologist’s suspicion can be raised by nodes >1 cm in short axis or >1.5 cm for jugulodigastric nodes (level II).34
Ultrasound elastography
Ultrasound elastography differentiates thyroid nodules based on elasticity and comes in two forms, strain and shear wave elastography.35,36 Many studies support the use of elastography;37-43 however there are limitations16 and it is not widely available. The AACE, ATA and KSThR recommend use of elastography as a supplementary study but not as a replacement for gray-scale ultrasound.13,16,19
CT and MRI of thyroid nodules
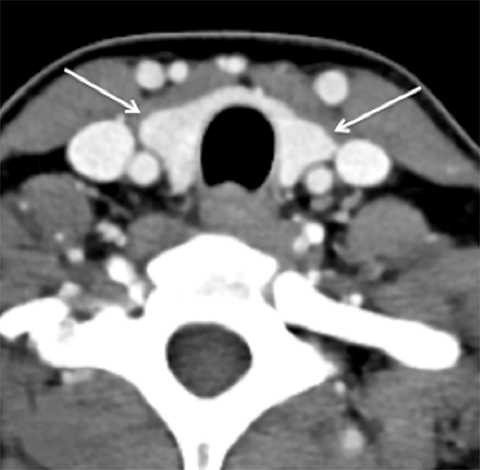
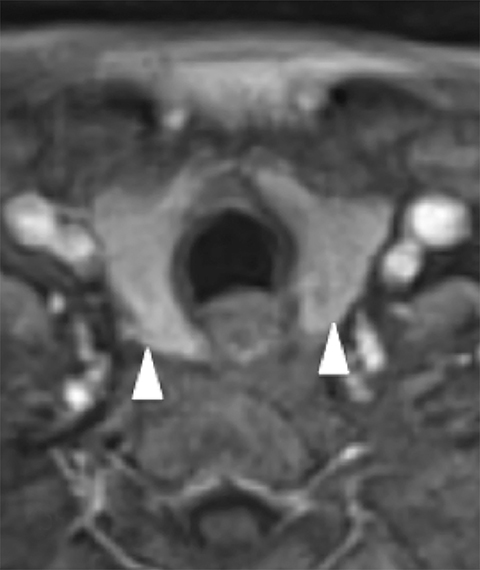
Cross-sectional imaging depicts the thyroid gland and its relationship to adjacent structures well. On non-contrast CT, the normal thyroid gland is homogeneously hyperattenuating relative to soft tissues in the neck due to its high iodine content. Following contrast administration, the thyroid enhances homogeneously and avidly because of its rich blood supply. On MRI, the thyroid gland is T1 hyperintense and T2 iso- to hypointense on noncontrast images and homogeneously enhances on post-gadolinium images (Figure 8).
Of note, iodinated contrast can interfere with the uptake of iodine-containing radionuclides, such as I-123 or I-131. Thus, timing of contrast-enhanced CT should be taken into consideration when diagnostic imaging or radionuclide ablation are planned. However, because iodine is cleared from the body within 4-8 weeks, nuclear imaging and therapy can be safely and successfully performed beyond this time period. If there is further concern about incomplete clearance, urine iodine sampling can be performed.44-46 Unlike CT contrast, MRI contrast (gadolinium) does not interfere with iodine uptake.47
Computed tomography and MRI are not the studies of choice for evaluating thyroid nodules because of poor spatial resolution, and the inability to detect features such as irregular margins and microcalcifications. However, the radiologist must be familiar with the reporting of thyroid nodules identified on cross-sectional imaging because of the frequency of studies including the neck and upper mediastinum (eg. neck and chest CTs) and the frequency of ITNs on these studies (up to 25% on chest CT48 and 16-18% on CT or MRI of the neck49,50). Apart from extra-thyroidal extension or lymphadenopathy, there are no reliable features that allow the radiologist to distinguish between benign and malignant thyroid nodules.51 Size by itself is also an unreliable feature, but is useful in guiding further work-up in conjunction with patient age.51
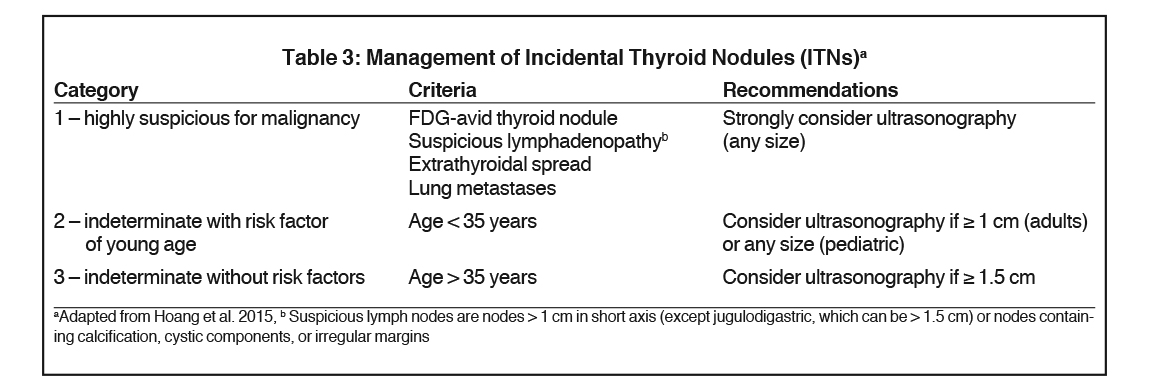
Not surprisingly, the reporting of ITNs can be highly variable.52-54 Fortunately, the three-tiered system proposed by Hoang et al. in 2012,55 supported by other literature49,56 and formalized in the ACR Incidental Thyroid Findings Committee white paper51 provides the radiologist with a systematic approach to managing ITNs identified on CT, MRI, and nuclear imaging, including FDG-PET. Further evaluation with thyroid ultrasound is recommended for three categories of ITN as follows:51,55
- Nodules with high risk characteristics such as lymphadenopathy, local invasion, or FDG avidity
- Nodules ≥1 cm in patients <35 years of age and
- Nodules ≥1.5 cm in patients >35 years of age
Ultrasonography of the neck in the evaluation of the carotid arteries, salivary glands, cervical lymph nodes, and other neck masses, can detect ITNs, as well. The sonographic features of the ITN should be described similarly to findings in a dedicated thyroid ultrasound. If there is insufficient evaluation of the thyroid, a full thyroid ultrasound should be recommended for complete characterization.51
Additional considerations in the reporting process include the presence of comorbidities and limited life expectancy that would increase the risk of treatment or increase the patient’s morbidity and mortality greater than a potential thyroid cancer. The ACR recommends that these patients do not undergo further evaluation.51
Nuclear imaging
The normal thyroid gland demonstrates homogeneous radiotracer uptake. Thyroid scintigraphy plays a role in the evaluation of a thyroid nodule in a patient who has low serum thyroid stimulating hormone levels. Thyroid scintigraphy with I-123 can identify a “hot” or hyperfunctioning nodule with radiotracer uptake greater than that of the surrounding thyroid. “Hot” nodules are rarely malignant and do not warrant cytologic analysis. A “warm” or iso-functioning nodule with radiotracer uptake equal to that of the surrounding thyroid, or a “cold” or hypofunctioning nodule with radiotracer uptake less than that of the surrounding thyroid, require further evaluation.57
Iodine-131 is useful as a therapeutic agent and imaging radionuclide. For diagnosis, I-131 is useful for whole body scanning to evaluate metastatic disease and for follow-up post-radioiodine ablation. High doses serve three purposes following thyroidectomy for malignancy: Ablate any remnant thyroid tissue, detect lymph node or distant metastases with high sensitivity, and ablate any tumor foci with uptake.34
Positron emission tomography with FDG is commonly performed in oncologic and non-oncologic settings. The normal thyroid gland has diffuse homogeneous low level FDG uptake similar to adjacent musculature. Incidental focal thyroid uptake occurs in 1-2% of cases58-60 with a reported malignancy rate of 11-14%.61,62 Due to this increased risk, the ACR and AACE recommend dedicated thyroid ultrasonography and FNA regardless of sonographic features19,51 whereas the ATA recommends sonographic and clinical evaluation of all FDG avid thyroid nodules and FNA of nodules >1 cm.16 There is no standard uptake value threshold that definitively distinguishes benign from malignant lesions.59
As mentioned previously, low-level FDG activity is normal. However increased diffuse radiotracer uptake occurs in 2% of patients.58 It typically reflects benign inflammatory conditions such as Hashimoto’s disease or other thyroiditis. Although thyroid nodules are rarely seen in these cases, the ATA recommends that diffuse uptake should prompt sonographic characterization.16
Conclusion
The incidence of thyroid cancer increased from 1975 to 2014 without a significant change in the mortality rate, most likely due to the earlier detection of indolent papillary thyroid cancers. Since the radiologist is often the first clinician to identify ITNs on cross-sectional imaging and is responsible for further characterization of nodules on ultrasonography, it is imperative that the radiologist be aware of the current data and recommendations with regards to thyroid nodule imaging. As described in this review, our recommendations are as follows:
Ultrasonography is the imaging modality of choice in the characterization of thyroid nodules because of its low cost, widespread availability, lack of ionizing radiation, ability to accurately depict nodule features, and ease of use for ultrasound-guided FNA.
Dedicated thyroid ultrasound should include a full survey of cervical lymph nodes.
Thyroid nodules are characterized by their location, size, composition, echogenicity, margins, orientation, calcifications, and vascularity. Benign features include predominantly cystic composition and an enlarged thyroid gland with multiple nodules. Irregular margins, taller-than-wide orientation, and microcalcifications are associated with malignancy. However, the overall pattern of sonographic features determines the risk of malignancy.
Risk stratification subsequently guides the radiologist recommendation for surveillance or FNA. Collaboration with the local referrers in your community may be helpful to standardize management recommendations.
We recommend the three-tiered approach to managing ITNs as described in the ACR Incidental Thyroid Findings Committee white paper (Table 3).51
References
- Roman BR, Morris LG, Davies L. The thyroid cancer epidemic, 2017 perspective. Curr Opin Endocrinol Diabetes Obes. 2017;24(5):332-336.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN. 2012 v1.0, Cancer Incidence and Mortality Worldwide. 2013; http://globocan.iarc.fr. Accessed December 3, 2017.
- Cancer Fast Stats. National Cancer Institute http://seer.cancer.gov/faststats/. Accessed November 26, 2017.
- Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295(18):2164-2167.
- Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014;140(4):317-322.
- Davies L, Morris LG, Haymart M, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: The increasing incidence of thyroid cancer. Endocr Pract. 2015;21(6):686-696.
- Morris LG, Tuttle RM, Davies L. Changing trends in the incidence of thyroid cancer in the United States. JAMA Otolaryngol Head Neck Surg. 2016;142(7):709-711.
- Kitahara CM, Sosa JA. The changing incidence of thyroid cancer. Nat Rev Endocrinol. 2016;12(11):646-653.
- Shi LL, DeSantis C, Jemal A, et al. Changes in thyroid cancer incidence, post-2009 American Thyroid Association guidelines. Laryngoscope. 2017;127(10):2437-2441.
- Hertzberg B, Middleton WD. Ultrasound: The Requisites. 3rd ed. St. Louis, MO: Elsevier; 2015:229-230.
- Danese D, Sciacchitano S, Farsetti A, et al. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8(1):15-21.
- Carmeci C, Jeffrey RB, McDougall IR, et al. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8(4):283-289.
- Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol. 2016;17(3):370-395.
- Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(4):1253-1263.
- Smith-Bindman R, Lebda P, Feldstein VA, et al. Risk of thyroid cancer based on thyroid ultrasound imaging characteristics: results of a population-based study. JAMA Intern Med. 2013;173(19):1788-1796.
- Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26(1):1-133.
- Kim JY, Lee CH, Kim SY, et al. Radiologic and pathologic findings of nonpalpable thyroid carcinomas detected by ultrasonography in a medical screening center. J Ultrasound Med. 2008;27(2):215-223.
- Frates MC, Benson CB, Charboneau JW, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237(3):794-800.
- Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules—2016 Update. Endocr Pract. 2016;22(5):622-639.
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Thyroid Carcinoma. 2017; https://www.nccn.org/professionals/physician_gls/pdf/thyroid.pdf. Accessed December 3, 2017.
- Tessler FN, Middleton WD, Grant EG, et al. ACR Thyroid imaging, reporting and data system (TI-RADS): White paper of the ACR TI-RADS Committee. J Am Coll Radiol. 2017;14(5):587-595.
- Ahn SS, Kim EK, Kang DR, et al. Biopsy of thyroid nodules: comparison of three sets of guidelines. AJR Am J Roentgenol. 2010;194(1):31-37.
- Peli M, Capalbo E, Lovisatti M, et al. Ultrasound guided fine-needle aspiration biopsy of thyroid nodules: Guidelines and recommendations vs clinical practice; a 12-month study of 89 patients. J Ultrasound. 2012;15(2):102-107.
- Hobbs HA, Bahl M, Nelson RC, et al. Applying the Society of Radiologists in Ultrasound recommendations for fine-needle aspiration of thyroid nodules: effect on workup and malignancy detection. AJR Am J Roentgenol. 2014;202(3):602-607.
- Tang AL, Falciglia M, Yang H, et al. Validation of American Thyroid Association ultrasound risk assessment of thyroid nodules selected for ultrasound fine-needle aspiration. Thyroid. 2017;27(8):1077-1082.
- Moon WJ, Jung SL, Lee JH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247(3):762-770.
- Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260(3):892-899.
- Nachiappan AC, Metwalli ZA, Hailey BS, et al. The thyroid: review of imaging features and biopsy techniques with radiologic-pathologic correlation. Radiographics. 2014;34(2):276-293.
- Brauer VF, Eder P, Miehle K, et al. Interobserver variation for ultrasound determination of thyroid nodule volumes. Thyroid. 2005;15(10):1169-1175.
- Kwak JY, Koo H, Youk JH, et al. Value of US correlation of a thyroid nodule with initially benign cytologic results. Radiology. 2010;254(1):292-300.
- Rosario PW, Purisch S. Ultrasonographic characteristics as a criterion for repeat cytology in benign thyroid nodules. Arq Bras Endocrinol Metabol. 2010;54(1):52-55.
- Asanuma K, Kobayashi S, Shingu K, et al. The rate of tumour growth does not distinguish between malignant and benign thyroid nodules. Eur J Surg. 2001;167(2):102-105.
- Park CJ, Kim EK, Moon HJ, et al. Thyroid nodules with nondiagnostic cytologic results: follow-up management using ultrasound patterns based on the 2015 American Thyroid Association guidelines. AJR Am J Roentgenol. 2017:1-6.
- King AD. Imaging for staging and management of thyroid cancer. Cancer Imaging. 2008;8(1):57-69.
- Kwak JY, Kim EK. Ultrasound elastography for thyroid nodules: recent advances. Ultrasonography. 2014;33(2):75-82.
- Shiina T, Nightingale KR, Palmeri ML, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 1: basic principles and terminology. Ultrasound Med Biol. 2015;41(5):1126-1147.
- Rago T, Santini F, Scutari M, et al. Elastography: new developments in ultrasound for predicting malignancy in thyroid nodules. J Clin Endocrinol Metab. 2007;92(8):2917-2922.
- Asteria C, Giovanardi A, Pizzocaro A, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18(5):523-531.
- Samir AE, Dhyani M, Anvari A, et al. Shear-wave elastography for the preoperative risk stratification of follicular-patterned lesions of the thyroid: diagnostic accuracy and optimal measurement plane. Radiology. 2015;277(2):565-573.
- Cappelli C, Pirola I, Gandossi E, et al. Real-time elastography: a useful tool for predicting malignancy in thyroid nodules with nondiagnostic cytologic findings. J Ultrasound Med. 2012;31(11):1777-1782.
- Choi WJ, Park JS, Koo HR, et al. Ultrasound elastography using carotid artery pulsation in the differential diagnosis of sonographically indeterminate thyroid nodules. AJR Am J Roentgenol. 2015;204(2):396-401.
- Nell S, Kist JW, Debray TP, et al. Qualitative elastography can replace thyroid nodule fine-needle aspiration in patients with soft thyroid nodules. A systematic review and meta-analysis. Eur J Radiol. 2015;84(4):652-661.
- Rago T, Scutari M, Santini F, et al. Real-time elastosonography: useful tool for refining the presurgical diagnosis in thyroid nodules with indeterminate or nondiagnostic cytology. J Clin Endocrinol Metab. 2010;95(12):5274-5280.
- Padovani RP, Kasamatsu TS, Nakabashi CC, et al. One month is sufficient for urinary iodine to return to its baseline value after the use of water-soluble iodinated contrast agents in post-thyroidectomy patients requiring radioiodine therapy. Thyroid. 2012;22(9):926-930.
- Sohn SY, Choi JH, Kim NK, et al. The impact of iodinated contrast agent administered during preoperative computed tomography scan on body iodine pool in patients with differentiated thyroid cancer preparing for radioactive iodine treatment. Thyroid. 2014;24(5):872-877.
- Nimmons GL, Funk GF, Graham MM, et al. Urinary iodine excretion after contrast computed tomography scan: implications for radioactive iodine use. JAMA Otolaryngol Head Neck Surg. 2013;139(5):479-482.
- Hoang JK, Branstetter BFt, Gafton AR, et al. Imaging of thyroid carcinoma with CT and MRI: approaches to common scenarios. Cancer Imaging. 2013;13(1):128-139.
- Ahmed S, Horton KM, Jeffrey RB, Jr., et al. Incidental thyroid nodules on chest CT: Review of the literature and management suggestions. AJR Am J Roentgenol. 2010;195(5):1066-1071.
- Nguyen XV, Choudhury KR, Eastwood JD, et al. Incidental thyroid nodules on CT: evaluation of 2 risk-categorization methods for work-up of nodules. AJNR Am J Neuroradiol. 2013;34(9):1812-1817.
- Youserm DM, Huang T, Loevner LA, et al. Clinical and economic impact of incidental thyroid lesions found with CT and MR. AJNR Am J Neuroradiol. 1997;18(8):1423-1428.
- Hoang JK, Langer JE, Middleton WD, et al. Managing incidental thyroid nodules detected on imaging: white paper of the ACR Incidental Thyroid Findings Committee. J Am Coll Radiol. 2015;12(2):143-150.
- Grady AT, Sosa JA, Tanpitukpongse TP, et al. Radiology reports for incidental thyroid nodules on CT and MRI: high variability across subspecialties. AJNR Am J Neuroradiol. 2015;36(2):397-402.
- Bahl M, Sosa JA, Nelson RC, et al. Imaging-detected incidental thyroid nodules that undergo surgery: a single-center experience over 1 year. AJNR Am J Neuroradiol. 2014;35(11):2176-2180.
- Hoang JK, Riofrio A, Bashir MR, et al. High variability in radiologists’ reporting practices for incidental thyroid nodules detected on CT and MRI. AJNR Am J Neuroradiol. 2014;35(6):1190-1194.
- Hoang JK, Raduazo P, Yousem DM, et al. What to do with incidental thyroid nodules on imaging? An approach for the radiologist. Semin Ultrasound CT MR. 2012;33(2):150-157.
- Bahl M, Sosa JA, Eastwood JD, et al. Using the 3-tiered system for categorizing workup of incidental thyroid nodules detected on CT, MRI, or PET/CT: how many cancers would be missed? Thyroid. 2014;24(12):1772-1778.
- Gharib H, Papini E. Thyroid nodules: clinical importance, assessment, and treatment. Endocrinol Metab Clin North Am. 2007;36(3):707-735, vi.
- Chen W, Parsons M, Torigian DA, et al. Evaluation of thyroid FDG uptake incidentally identified on FDG-PET/CT imaging. Nucl Med Commun. 2009;30(3):240-244.
- Nishimori H, Tabah R, Hickeson M, et al. Incidental thyroid “PETomas”: clinical significance and novel description of the self-resolving variant of focal FDG-PET thyroid uptake. Can J Surg. 2011;54(2):83-88.
- Soelberg KK, Bonnema SJ, Brix TH, et al. Risk of malignancy in thyroid incidentalomas detected by 18F-fluorodeoxyglucose positron emission tomography: a systematic review. Thyroid. 2012;22(9):918-925.
- Kwak JY, Kim EK, Yun M, et al. Thyroid incidentalomas identified by 18F-FDG PET: sonographic correlation. AJR Am J Roentgenol. 2008;191(2):598-603.
- Choi JS, Choi Y, Kim EK, et al. A risk-adapted approach using US features and FNA results in the management of thyroid incidentalomas identified by 18F-FDG PET. Ultraschall Med. 2014;35(1):51-58.
Citation
R C, D K.Imaging of thyroid nodules. Appl Radiol. 2019; (1):16-26.
February 6, 2019