Imaging blunt pancreatic and duodenal trauma
Images
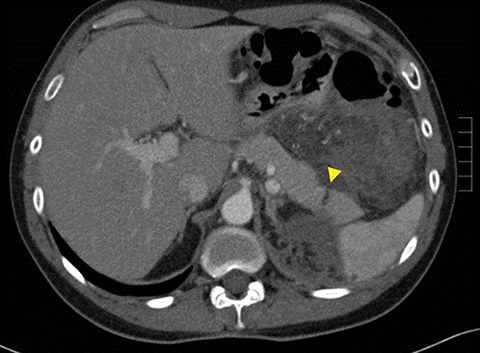
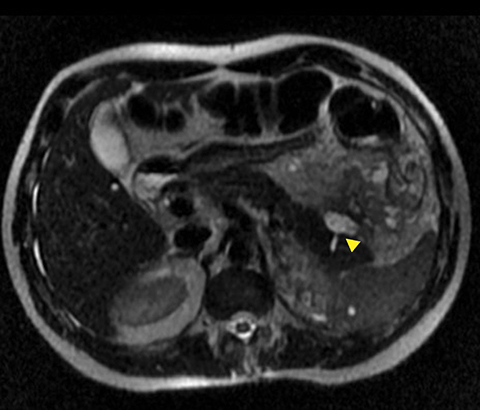
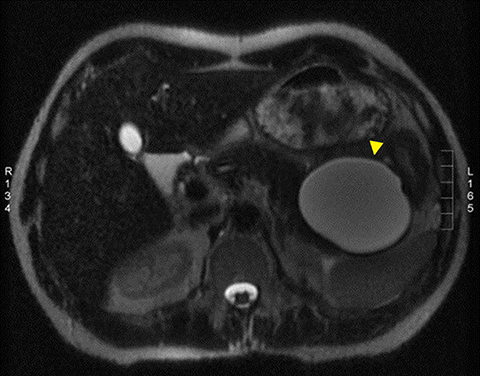
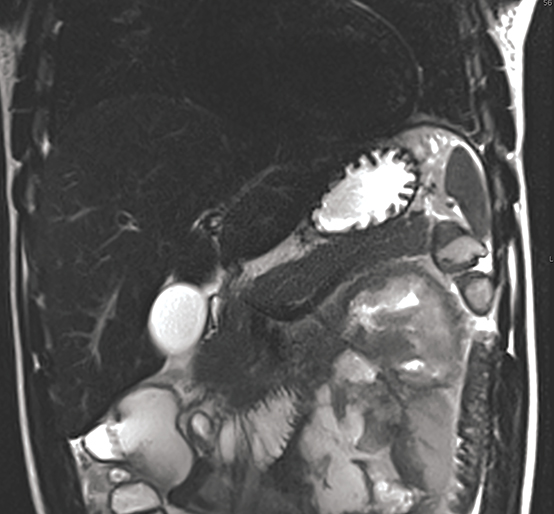
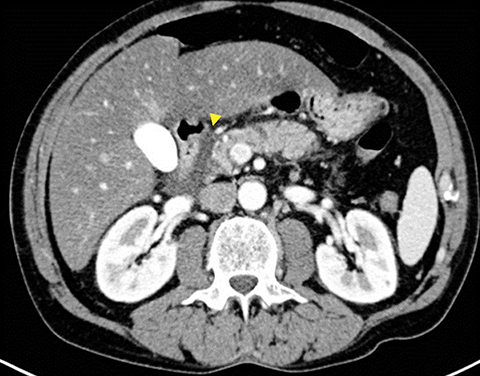
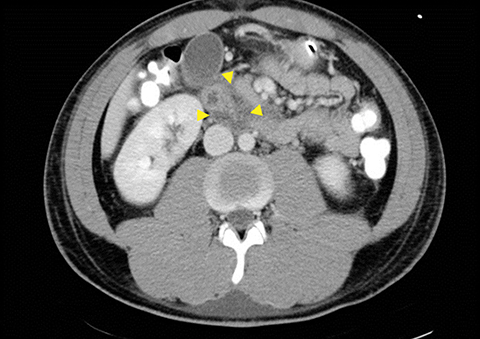
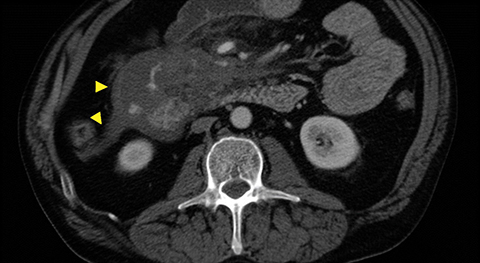
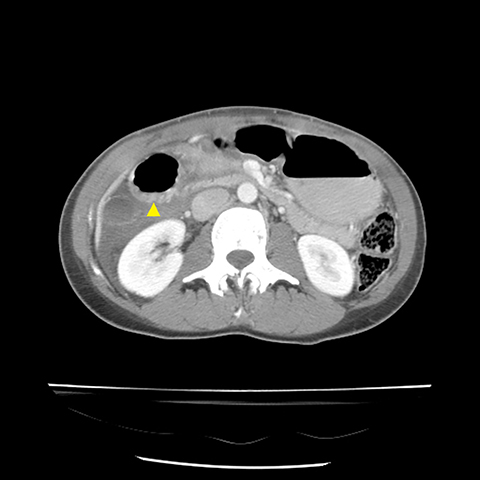
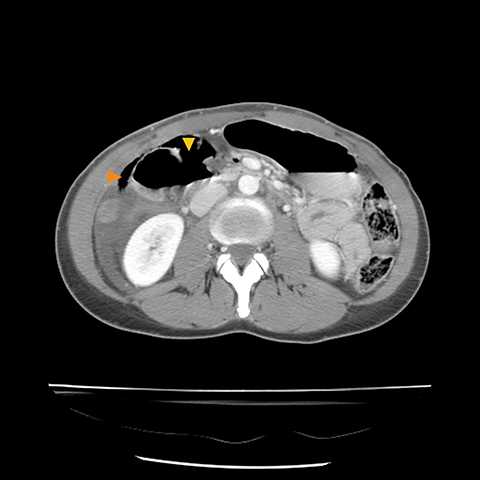
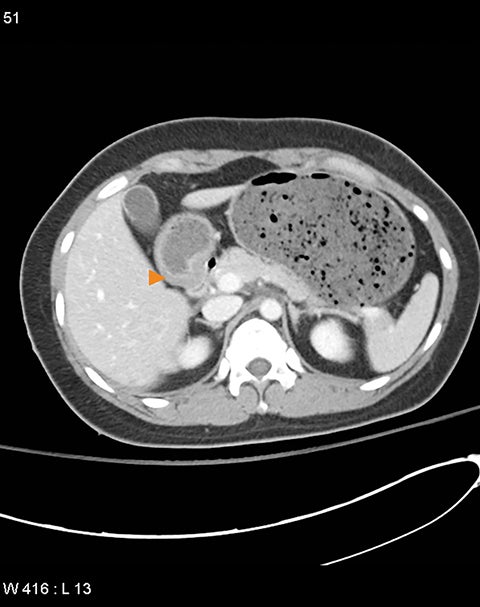
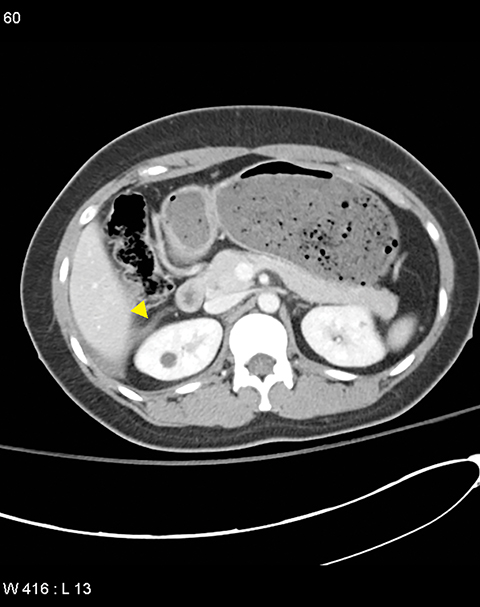

The incidence of pancreatic injury in blunt abdominal trauma ranges from 0.2 to 12%, with mortality rates as high as 30%.1 The pancreas and duodenum are commonly injured simultaneously, with an incidence of 50-98%, often also involving the left hepatic lobe and spleen.2-6 Isolated pancreatic injuries are rare, occurring with an incidence of less than 30%.2-6 Mortality and morbidity significantly increase when the diagnosis is not recognized at admission. Thus, a prompt diagnosis of pancreatic injury is critical in delivering appropriate and timely interventions and for minimizing complications.7 Computed tomography (CT) is the imaging modality of choice in stable patients and provides an excellent means for detecting and characterizing solid visceral injuries.8
Mechanism of injury
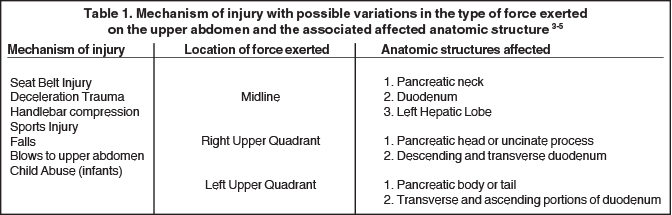
Blunt pancreatic injury (BPI) occurs with compression of the pancreas against the first and second lumbar vertebrae secondary to upper/mid-abdomen frontal impacts. Pancreatic injury is more common in children and adolescents secondary to paucity of intra-abdominal fat and a thinner layer of peripancreatic fat, which provide protection from blunt trauma.3-5 Greater than 65% of blunt pancreatic injuries occur in the pancreatic body, to the left of the superior mesenteric vessels.3-5 Due to the retroperitoneal location of the pancreas and its proximity to other viscera and major vascular structures, pancreatic injury is commonly associated with injury to multiple abdominal organs and vascular structures (Table 1) in 50-100% of patients with BPI.3-5
Clinical presentation
History in the trauma setting may not be helpful and physical examination is generally nonspecific, but pancreatic injury should always be considered in patients with epigastric tenderness.9 Grey Turner’s sign and Cullen’s sign may be present, although these are not sensitive markers of pancreatic trauma.9 Absence of such clinical findings does not exclude pancreatic injury in a patient with a typical mechanism of injury because pancreatic injury often evolves over time with activation of pancreatic enzymes and initiation of the inflammatory response.
Complications
Early complications arise primarily from concurrent injuries to other organs, predominantly vascular causing hemorrhage. Late complications or morbidity is increased in patients with delayed diagnoses of pancreatic injury, especially those with a missed ductal injury. Damage to the main pancreatic duct occurs in 15% of cases and is crucial to ascertain before or during laparotomy, as it necessitates pancreatic reconstruction by an experienced hepatobiliary surgeon.9 In patients with pancreatic trauma who have survived the initial 48–72 hours, leakage of corrosive pancreatic juice from an undiagnosed injured duct causes peripancreatic inflammation and may result in sepsis, which is the major cause of morbidity and mortality.
A pancreatic fistula develops in 2% to 15% of patients with pancreatic injury.10 Fistulae are especially common after proximal pancreatic injuries and combined pancreaticoduodenal injuries.11 Pancreatic abscess occurs in 10% to 25% of patients following pancreatic injury.11 Pancreatic ductal injury and colonic injury are independent predictors of abscess formation following pancreatic trauma.12 Pancreatic abscess can be a lethal complication, with mortality rates as high as 20% to 27%.13 Pancreatic pseudocysts occur in 1.6% to 4% of patients following pancreatic injury.12,14 Pseudocysts are most likely to form after missed or inadequately treated ductal injury (Figure 1). Furthermore, the rate of pseudocyst formation following nonoperative management of pancreatic injuries may be as high as 30% to 44%.15 Pancreatitis complicates 3% to 8% of pancreatic injuries and should be suspected in patients with persistent abdominal pain, nausea, vomiting and hyperamylasemia.16 Duodenal hematomas typically occur in younger patients. Blood accumulates in the submucosal or subserosal layer of the otherwise intact duodenal wall. Gastric outlet obstruction is a common complication of duodenal hematomas.5
Imaging of blunt pancreatic injury
Role of imaging
Because of the retroperitoneal location of the pancreas, the initial physical examination, diagnostic peritoneal lavage (DPL) and ultrasonography are relatively insensitive in detecting pancreatic injury. Furthermore, elevations in serum amylase and lipase are not reliable determinants of early pancreatic injury. In many cases they are normal even with high-grade injury.9 Sensitivity of multidetector CT (MDCT) for detection of pancreatic injuries has been reported between 70% and 95%.17 Thus, mechanism alone is considered a strong indication for CT.6 With the evolution in technology, CT now offers faster scan times and better temporal and spatial resolution, which is ideal to evaluate the retroperitoneum and detect delayed complications of pancreatic injury.18 In situations when CT is not available, persistently elevated or rising serum amylase and lipase 6 hours after trauma can be a reliable and cost-effective screening tool.19
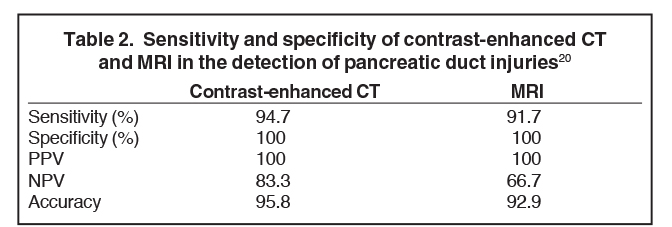
Pancreatic duct injury is the main prognostic indicator of clinical outcome in patients with blunt traumatic injury. However, pancreatic duct injury is poorly characterized on CT and it is often detected during laparotomy.9 Diagnosing a pancreatic duct injury remains a significant challenge in the management of pancreatic trauma. Lacerations less than 50% of the diameter of the pancreas usually indicate no ductal injury.9 In a recent prospective study by A. Panda et al, MR performed equally well in grading pancreatic injury and identifying pancreatic duct injury when compared to contrast-enhanced CT (CECT, Table 2) with good intermodality agreement.20 Thus, magnetic resonance cholangiopancreaticography (MRCP) or endoscopic retrograde cholangiopancreaticography (ERCP) can be used for further assessment if ductal injury is suspected.20,21 MRCP, however, may demonstrate pancreatic parenchymal abnormalities not visualized on ERCP and aid ERCP-guided therapy when ductal abnormalities are present.4,5,18
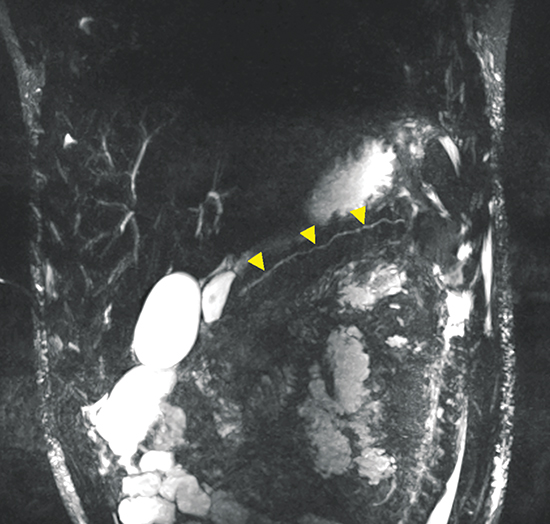
MRCP, in combination with the intravenous administration of secretin, has been successfully employed to improve characterization of pancreatic ductal anatomy, demonstrate ductal disruptions beyond an obstructed duct (unlike ERCP) and provide dynamic information about ongoing leakage.22,23 An MRCP-facilitated secretin test can be performed with a dynamic, breath-hold, two-dimensional single-shot RARE heavily T2-weighted sequence, along the coronal plane. No post-processing is required. Secretin given as a synthetic agent intravenously, improves visualization of the pancreatic duct (Figure 2) by increasing the caliber of the duct almost immediately and peaking between 2 and 5 minutes.24,25
For trauma patients already in the operating room for concomitant injuries, or those not eligible for MRCP or when ERCP is not available, intraoperative US may be performed to assist with immediate diagnosis of ductal injury.26 However, there are no large-scale studies that compare the sensitivity and specificity of US for pancreatic ductal injuries to those of ERCP, MRCP or cholecystocholangiopancreatography.
Imaging techniques
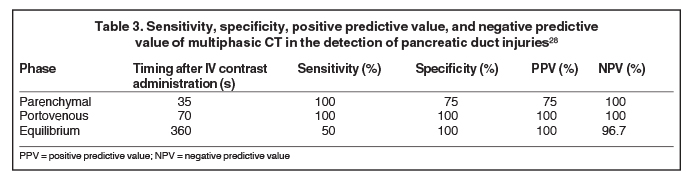
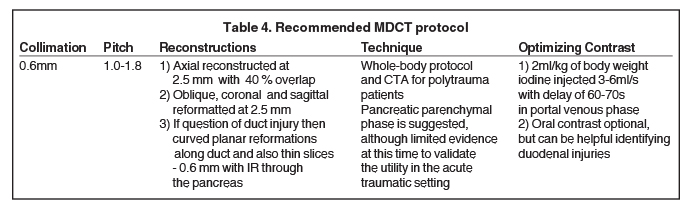
With the availability of 64-slice and higher CT scanners, the entire scan can be acquired in the arterial phase. This facilitates optimal detection of vascular injury.27 Wong et al (2008) concluded that the portal venous phase CT was the most accurate scan in detecting pancreatic duct injuries (Table 3).28 Thin sections are routinely acquired for better visualization of the main pancreatic duct (Table 4), which normally measures 3mm to 4mm.27 3D post-processing techniques, such as curved planar reformats, appear to improve detection and characterization of pancreatic lacerations.27,29 However, further investigation is needed to confirm the utility of these techniques in the trauma setting.
Imaging findings
The injured pancreas may appear normal on CT images, particularly in the first 12 hours after trauma.9,17 Sensitivity of CT may improve with time after injury, as tissue damage from activated pancreatic secretions and peripancreatic inflammation evolves over time. Therefore, a repeat CT in 24-48 hours may be warranted for patients with persistent symptoms.17,18 Published data demonstrates poor sensitivity for pancreatic injury and pancreatic ductal injury even with advanced CT technology.30 Thus, correlating the mechanism of injury and recognizing the subtle signs on CT are crucial to early and accurate diagnosis of pancreatic trauma. The CT findings of pancreatic trauma can be categorized into direct (specific) and indirect (nonspecific) features.
Direct CT findings
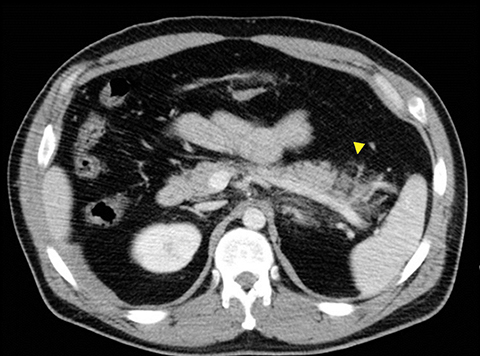
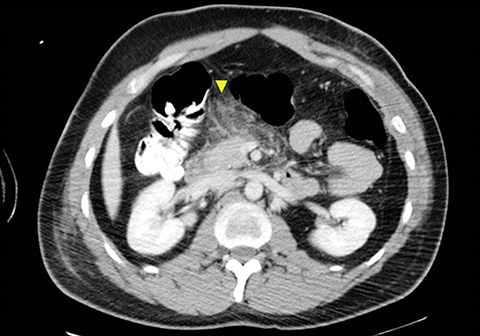
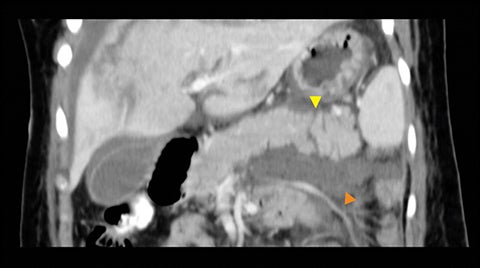
The direct findings include 1) laceration; 2) transection; and 3) focal pancreatic enlargement or hematoma. Laceration that extends through the entire pancreas is termed transection or fracture. Lacerations can be further divided into superficial (involving <50% of parenchymal tissue) and deep (>50% parenchymal tissue).4, 9 Using this cutoff, deep lacerations have been associated with main pancreatic duct injury.4,17,31 Given their high specificity, direct CT findings should signal further diagnostic or therapeutic intervention. Correlation between grade of injury and morbidity with complication rates was demonstrated by Smego et al.32 Grading of injury allows accurate description and localization, as well as determination of surgical versus non-operative treatment. Modified CT grading system of pancreatic injuries by Wong et al (2008) correlates well with AAST grading.28
Grade I and II injuries indicate contusion and/or superficial laceration without ductal injury (Figures 3 and 4). Grade III indicates deep laceration or transection with duct injury to the left of SMV (Figure 5). Grade IV injury indicates deep laceration or transection with duct injury to the right of SMV (Figure 6). Grade V injury is the most severe; it indicates pancreatic head disruption and is frequently associated with concomitant duodenal injury (Figure 7).
Indirect CT findings
The indirect findings on CT that may indicate pancreatic injury include: 1) peripancreatic fluid in the lesser sac; 2) thickening of the left anterior renal fascia; and 3) fluid interdigitating between the pancreas and the spleen. The indirect CT finding of peripancreatic fluid is highly sensitive (67-85%) but nonspecific, as it can be seen in trauma to other organs.31 However, the presence of indirect findings necessitates close examination of the pancreas and surrounding organs.
Pitfalls in imaging
Despite improved image quality with MDCT, pitfalls remain in assessing pancreatic injury through imaging. Concurrent pancreatic atrophy in the elderly and those with chronic pancreatic pathology may make identifying ductal injury more difficult.28 False-negative findings may arise in patients with minimal retroperitoneal fat, leading to decreased contrast between abdominal organs and making subtle defects more difficult to identify.1,27 In addition, hemorrhage resulting from concurrent organ injury can mask pancreatic laceration.1,27 Likewise, a laceration can be obscured by close apposition of the pancreatic fragments.31
On the contrary, false-positives may result due to normal morphologic variations of pancreatic architecture that can be misread on imaging as pancreatic injury. Firstly, the pancreas often contains numerous clefts that may be difficult to differentiate from lacerations and may lead to unnecessary further work-up.31 Secondly, patients with fatty atrophy of the pancreas, a relatively common finding in the obese and elderly, have imaging findings that may mimic a pancreatic laceration.27 Similarly, asymmetric fatty replacement may mimic contusions.33 Lastly, small amounts of fluid tracking between lobules, which can arise secondary to aggressive fluid resuscitation, can be mistakenly interpreted as small lacerations.4
Managment
Nonoperative management has become the preferred treatment for hemodynamically stable patients with BPI largely because MDCT/CT angiography is now highly accurate in excluding surgically important vascular and solid organ injuries. However, pancreatic injuries are still commonly missed with subsequent delayed diagnosis, which correlates with increased complications and adverse outcomes.27
The location of injury (proximal versus distal), involvement of the pancreatic duct and overall status of the patient are major determinants of appropriate management in BPI.30 Nonoperative management has been proposed in the management of Grade I and II pancreatic injuries. It consists of bowel rest with nasogastric drainage and total parenteral nutrition, serial abdominal examination, and serial serum amylase determination with laparotomy or further imaging studies performed for worsening abdominal examination or persistent hyperamylasemia. However, nonoperative management in the setting of main pancreatic ductal injury leads to a high incidence of late complications, in particular pseudocysts and pancreatic fistula.34
For distal ductal injury (grade III) , more conservative management protocols utilizing external drainage and distal pancreatectomy has been utilized more recently with reported lower mortality and morbidity rates compared to more radical procedures such as pancreaticoenteric anastomosis.30,35,36 On the other hand, proximal pancreatic ductal injuries (Grades IV and V) are more difficult to manage with little data in the literature to suggest the optimal approach. Patient with grade IV and V injuries frequently get a pancreatic stent and Whipple’s procedure, respectively. Recent trends, however, favor more conservative management, such as closed-suction drainage alone.30
Imaging in blunt duodenal injury
CT Findings
CT is used to grade duodenal injury (Table 5). Duodenal injuries can be divided into minor and major categories. Minor injuries include duodenal hematoma and partial thickness lacerations, whereas major injuries include transmural lacerations/perforations and massive disruption of the duodeno-biliary-pancreatic complex.2,5 Findings suggestive of duodenal injury include: 1) wall thickening of > 4mm; 2) lack of wall continuity; 3) peri-duodenal fluid; 4) fluid in the right anterior pararenal space; 5) diminished bowel-wall enhancement; 6) extraluminal air or contrast (Figures 8, 9). The key findings to remember include lack of wall continuity and extraluminal air or contrast, which indicate duodenal perforation (Figure 8B).
Imaging pitfalls
A few causes of false-positive findings in blunt duodenl injury imaging include: 1) duodenal diverticulum simulating retroperitoneal air, 2) retroperitoneal hematoma from a nonduodenal source, and 3) unopacified bowel loops adjacent to the duodenum which may obscure subtle findings.
Management
Isolated duodenal hematomas are managed conservatively except in patients with acute abdomen, sepsis or uncontrolled bleeding.5,37 Surgical management of duodenal lacerations hinges on the extent and severity of the duodenal injury, as well as the involvement of the adjacent vasculature, biliary tree, and pancreas.5,6,38 Uncomplicated duodenal lacerations are repaired by primary surgical closure, known as duodenorrhaphy, while more complex injuries require reconstructive procedures. Pancreaticoduodenectomy is reserved for patients with severe combined duodenal and pancreatic head injuries or injuries to segments D1 and D2 due to their common blood supply with the pancreatic head.5 Lower duodenal (D3 and D4) injuries can be treated similarly to small-bowel injury (resection and reanastomosis).6,38
Conclusion
Pancreaticoduodenal injury in blunt abdominal trauma is uncommon, but when present is associated with high mortality. Mortality and morbidity significantly increase when this injury is not recognized at admission. Therefore, an early diagnosis is critical in delivering appropriate and timely interventions. CT is the imaging modality of choice in stable patients. Radiologists must be aware, however, of the imaging pitfalls that may confound CT findings. Moreover, pancreatic duct injury is poorly characterized on CT and remains a significant challenge in the management of pancreatic trauma. Lately, MRCP in combination with the intravenous administration of secretin has been successfully employed to improve characterization of pancreatic ductal anatomy. A modified CT grading system of pancreatic injuries by Wong et al (2008) allows for determination of surgical versus nonoperative treatment. Nonoperative management of pancreaticoduodenal injuries has become the preferred treatment for hemodynamically stable patients due to increased accuracy of MDCT in excluding other surgically important vascular and solid organ injuries.
References
- Cirillo RL Jr, Koniaris LG. Detecting blunt pancreatic injuries. J Gastrointest Surg. 2002;6(4):587-598.
- Daly KP, Ho CP, Persson DL,et al. Traumatic retroperitoneal injuries: review of multidetector CT findings. Radiographics. 2008;28(6):1571-1590.
- Linsenmaier U, Wirth S, Reiser M, et al. Diagnosis and classification of pancreatic and duodenal injuries in emergency radiology. Radiographics. 2008;28(6):1591-1602.
- Rekhi S, Anderson SW, Rhea JT, et al. Imaging of blunt pancreatic trauma. Emerg Radiol. 2010; 17(1):13-19.
- Soto JA, Anderson SW. Multidetector CT of blunt abdominal trauma. Radiology. 2012;265(3): 678-693.
- Stawicki SP, Schwab CW. Pancreatic trauma: demographics, diagnosis, and management. Am Surg. 2008; 74(12);1133-1345.
- Wolf A, Bernhardt J, Patrzyk M, et al. The value of endoscopic diagnosis and the treatment of pancreas injuries following blunt abdominal trauma. Surg Endosc. 2005;19(5):665-669.
- Peitzman AB, Makaroun MS, Slasky BS, et al. Prospective study of computed tomography in initial management of blunt abdominal trauma. J Trauma. 1986;26(7):585-592.
- Lahiri R, Bhattacharya S. Pancreatic trauma. Ann R Coll Surg Engl. 2013;95(4):241-245.
- Iqbal CW, St. Peter SD, Tsao K, et al. Operative vs nonoperative management for blunt pancreatic transection in children: multi-institutional outcomes. J Am Coll Surg. 2014;218(2):157-162.
- Jones C. Management of pancreatic trauma. Am J Surg. 1985;150(6):698-704.
- Patton JH Jr, Lyden SP, Croce MA, et al. Pancreatic trauma: a simplified management guideline. J Trauma. 1997; 43(2):234-239; discussion 239-241.
- Feliciano DV, Martin TD, Cruse PA, et al. Management of combined pancreatoduodenal injuries. Ann Surg. 1987; 205(6):673-680.
- Vasquez JC, Coimbra R, Hoyt DB, et al. Management of penetrating pancreatic trauma: an 11-year experience of a level-1 trauma center. Injury. 2001;32(10):753-759.
- Wales PW, Shuckett B, Kim PC. Long-term outcome after nonoperative management of complete traumatic pancreatic transection in children. J Pediatr Surg. 2001;36(5):823-827.
- Lin BC, Chen RJ, Chang JF, et al. Management of blunt major pancreatic injury. J Trauma. 2004; 56(4):774-778.
- Wong YC, Wang LJ, Lin BC, et al. CT grading of blunt pancreatic injuries: prediction of ductal disruption and surgical correlation. J Comput Assist Tomogr. 1997;21(2):246-250.
- Gupta A, Stuhlfaut JW, Fleming KW, et al. Blunt trauma of the pancreas and biliary tract: a multimodality imaging approach to diagnosis. Radiographics. 2004;24(5):1381-1395.
- Mahajan A, Kadavigere R, Sripathi S, et al. Utility of serum pancreatic enzyme levels in diagnosing blunt trauma to the pancreas: a prospective study with systematic review. Injury. 2014;45(9):1384-1393.
- Panda A, Kumar A, Gamanagatti S, et al. Evaluation of diagnostic utility of multidetector computed tomography and magnetic resonance imaging in blunt pancreatic trauma: a prospective study. Acta Radiol. 2015; 56(4):387-396.
- Wright MJ, Stanski C. Blunt pancreatic trauma: a difficult injury. South Med J. 2000;93(4):383-385.
- Gillams AR, Kurzawinski T, Lees WR. Diagnosis of duct disruption and assessment of pancreatic leak with dynamic secretin-stimulated MR cholangiopancreatography. AJR Am J Roentgenol. 2006;186(2):499-506.
- Sanyal R, Stevens T, Novak E, et al. Secretin-enhanced MRCP: review of technique and application with proposal for quantification of exocrine function. AJR Am J Roentgenol. 2012;198(1):124-132.
- Griffin N, Charles-Edwards G, Grant LA. Magnetic resonance cholangiopancreatography: the ABC of MRCP. Insights Imaging. 2012;3(1):11-21.
- Ragozzino A, Manfredi R, Scaglione M, et al. The use of MRCP in the detection of pancreatic injuries after blunt trauma. Emerg Radiol. 2003;10(1):14-18.
- Hofmann LJ, Learn PA, Cannon JW. Intraoperative ultrasound to assess for pancreatic duct injuries. J Trauma Acute Care Surg. 2015; (4):888-891.
- Dreizin D, Bordegaray M, Tirada N, et al. Evaluating blunt pancreatic trauma at whole body CT: current practices and future directions. Emerg Radiol. 2013;20(6):517-527.
- Wong YC, Wang LJ, Fang JF, et al. Multidetector-row computed tomography (CT) of blunt pancreatic injuries: can contrast-enhanced multiphasic CT detect pancreatic duct injuries? J Trauma. 2008;64(3):666-672.
- Gong JS, Xu JM. Role of curved planar reformations using multidetector spiral CT in diagnosis of pancreatic and peripancreatic diseases. World J Gastroenterol. 2004;10(13):1943-1947.
- Potoka DA, Gaines BA, Leppaniemi A, et al. Management of blunt pancreatic trauma: what’s new? Eur J Trauma Emerg Surg. 2015;41(3):239-250.
- Gordon RW, Anderson SW, Ozonoff A, et al. Blunt pancreatic trauma: evaluation with MDCT technology. Emerg Radiol. 2013;20(4):259-266.
- Smego DR, Richardson JD, Flint LM. Determinants of outcome in pancreatic trauma. J Trauma. 1985;25(8):771-776.
- Katz DS, Hines J, Math KR, et al. Using CT to reveal fat-containing abnormalities of the pancreas. AJR Am J Roentgenol. 1999;172(2):393-396.
- Wind P, Tiret E, Cunningham C, et al. Contribution of endoscopic retrograde pancreatography in management of complications following distal pancreatic trauma. Am Surg. 1999;65(8):777-783.
- Beres AL, Wales PW, Christison-Lagay ER, et al. Non-operative management of high-grade pancreatic trauma: is it worth the wait? J Pediatr Surg. 2013;48(5):1060-1064.
- Sharpe JP, Magnotti LJ, Weinberg JA, et al. Impact of a defined management algorithm on outcome after traumatic pancreatic injury. J Trauma Acute Care Surg. 2012;(1):100-105.
- Strobel O, Schneider L, Philipp S, et al. Emergency pancreatic surgery-demanding and dangerous. Langenbecks Arch Surg. 2015 online.
- Degiannis E, Boffard K. Duodenal injuries. Br J Surg. 2000;87(11):1473-1479.
Citation
S S, F K, Si R, P M, L L, S N. Imaging blunt pancreatic and duodenal trauma. Appl Radiol. 2016; (11):22-28.
November 2, 2016