How to perform whole-body PET/MRI on pediatric patients
Performing a whole-body integrated positron emission tomography (PET)/magnetic resonance imaging (MRI) examination on a pediatric patient can be challenging, especially since integrated PET/MRI scanners are not yet in widespread use. Radiologists at Stanford University in Stanford, CA, have been early adopters of the technology and were among the first to use the system for children in North America. A team of Stanford pediatric radiologists share a step-by-step protocol for staging of children with cancer in the Journal of Visualized Experiments.
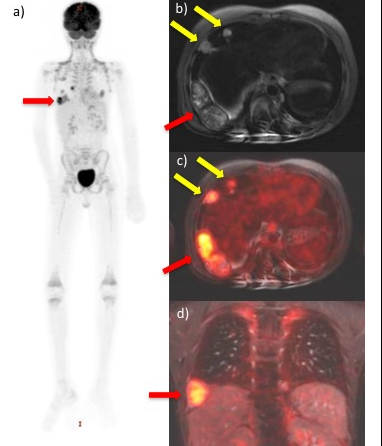
18F-FDG PET/MR scan of a child with metastasized
undifferentiated sarcoma. A) Maximum Intensity Projection
(MIP) of 18F-FDG PET scan of the whole body shows
multiple metastases in the chest. B) Axial T2-weighted
scan shows a pleural-based mass (red arrow) and multiple
pulmonary metastases (yellow arrows). C) Axial T2-weighted
scan co-registered with color-encoded FDG-PET shows
multiple hypermetabolic metastases (arrows). D) Coronal
ferumoxytol-enhanced gradient echo scan co-registered
with 18F-FDG PET shows pleural-based hypermetabolic
mass (red arrow).
PET/MRI scanners align the features of MRI that include excellent soft tissue contrast visualization, dynamic contrast-enhanced imaging, and diffusion-weighted imaging with PET’s ability to provide quantitative physiologic and metabolic data. The major benefits of PET/MRI scans include potentially more accurate diagnosis and treatment options than PET/computed tomography (CT) provides as well as the convenience of having two scans performed in a imaging suite during a single appointment. For small children, this procedure can avoid duplicate sedation. Most importantly, an integrated PET/MRI scan exposes patients to about 50-74% less radiation than a PET/CT scan, and therefore improves patient safety, especially for children.1
Heike E. Daldrup-Link, MD, PhD, professor of radiology and director of the Pediatric Molecular Imaging Program, and co-authors, developed a step-by-step protocol for the acquisition of whole-body 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) PET/MRI data of pediatric cancer patients. The objective of the protocol is to acquire high quality diagnostic images while keeping the scan time to a minimum. The authors state that the protocol’s most critical aspect is time-efficient planning and integration of PET-slabs and MRI sequences with the correct parameters and in the most efficient consecutive order so that acquisition of images of the entire body is continuous. They caution that this is not a “one size fits all” approach and that radiologists need to tailor PET and MRI scan parameters to each individual patient.
Topics of the protocol include tips for optimized workflows, including advance patient safety screening, patient preparation for the exam, as well as suggested child-tailored image acquisition protocols for both whole-body staging and evaluation of the primary tumor in one session. Using the iron supplement ferumoxytol “off label” as a MR contrast agent avoids gadolinium chelate administration and eliminates concerns for potential gadolinium deposition in the brain.
The authors also describe techniques for efficient image transfer to the picture archiving and communications system (PACS), image data processing, image analysis, and standard uptake alues (SUV) measurements.When following these protocols, the authors state that a typical scan will take 60-90 minutes depending on the location and size of the primary tumor and the length of the patient.
Co-authors and postdoctoral research fellows Anuj Pareek and Anne M. Muehe spent over two years working on and perfecting this protocol, Dr. Daldrup-Link told Applied Radiology. She said, “We originally developed the protocol for children typically 6 years and older, who are able to comply with breath-hold instructions. However, the protocol can also be used with younger sedated children by either using breath-hold MRI sequences for anatomical orientation or by using ultrafast sequences with free breathing acquisition modes.”
“The Stanford team found that ferumoxytol-enhanced PET/MR scans provided equal or superior tumor staging results compared to clinical standard tests in more than 50% of evaluated patients. Novel whole body PET/MR staging tests can impact health care economics and patient care by providing more efficient ‘one stop’ staging exams, saving our most vulnerable patients from radiation exposure, and eliminating associated risks of potential long-term effects later in life,” she said.
A 10-minute long video of the procedure may be watched by clicking here.
REFERENCES
- Muehe AM, Theruvath AJ, Lai L, et al. How to Provide Gadolinium-Free PET/MR Cancer Staging of Children and Young Adults in Less than 1 h: the Stanford Approach. Mol Imaging Biol. 2017 Published online July 18, 2017. Doi: 10.1007/s11307-017-1105-7.
- Pareek A, Muehe AM, Theruvath AJ, et al. Whole-body PET/MRI of Pediatric Patients: The Details That Matter. J Vis Exp. 2017 e57128. Doi: 10.3791/57128.