Hereditary Angioedema
Images
Case Summary
A teenager with a known personal and family history of hereditary angioedema (HAE) presented to the emergency department with generalized abdominal pain, constipation, and nonbilious, non-bloody emesis for several days without signs of obstruction. On physical examination there was generalized abdominal pain without peritoneal signs. Laboratory studies demonstrated mild leukocytosis (14 × 109/L) but were otherwise within normal limits.
Imaging Findings
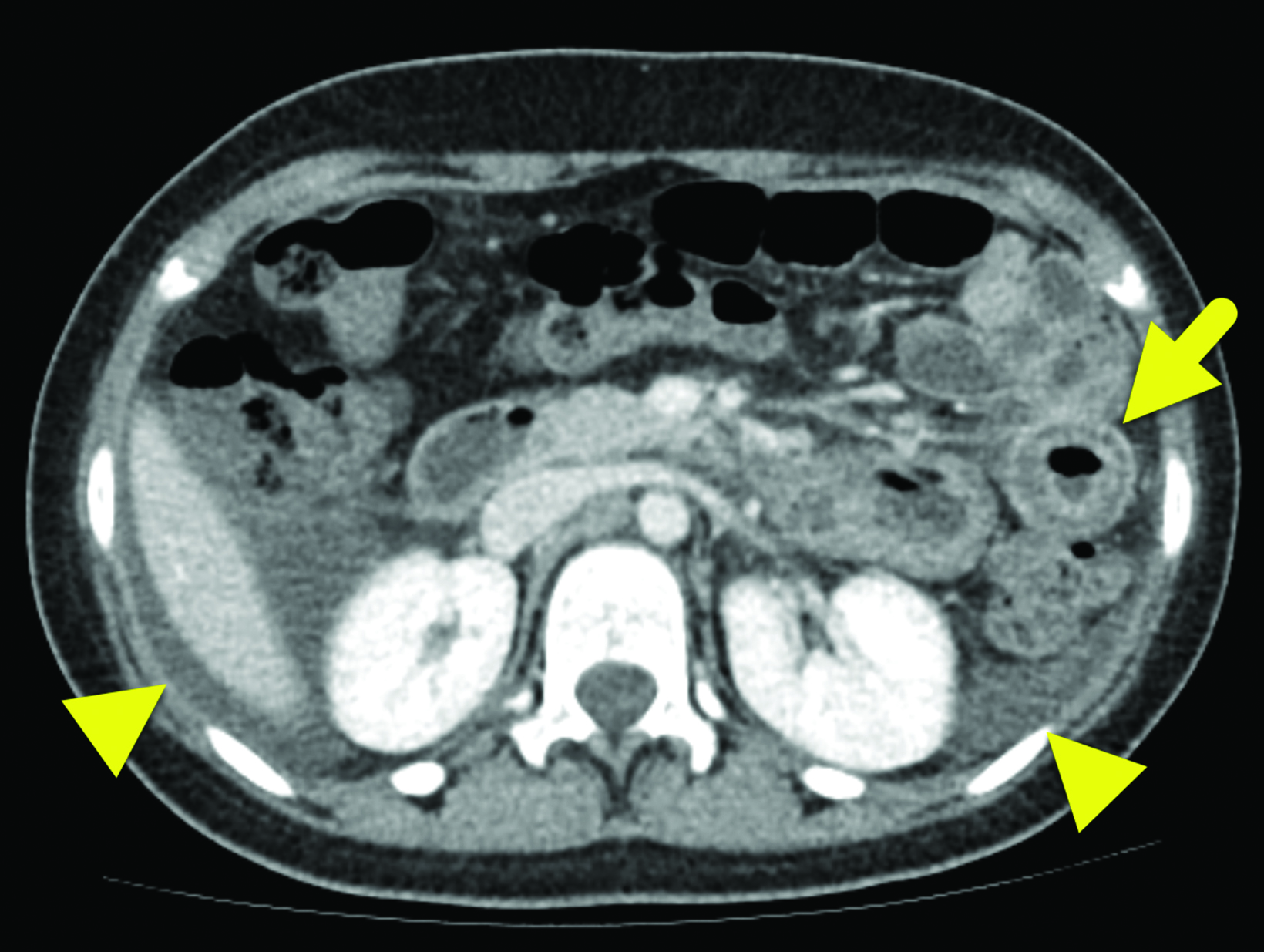
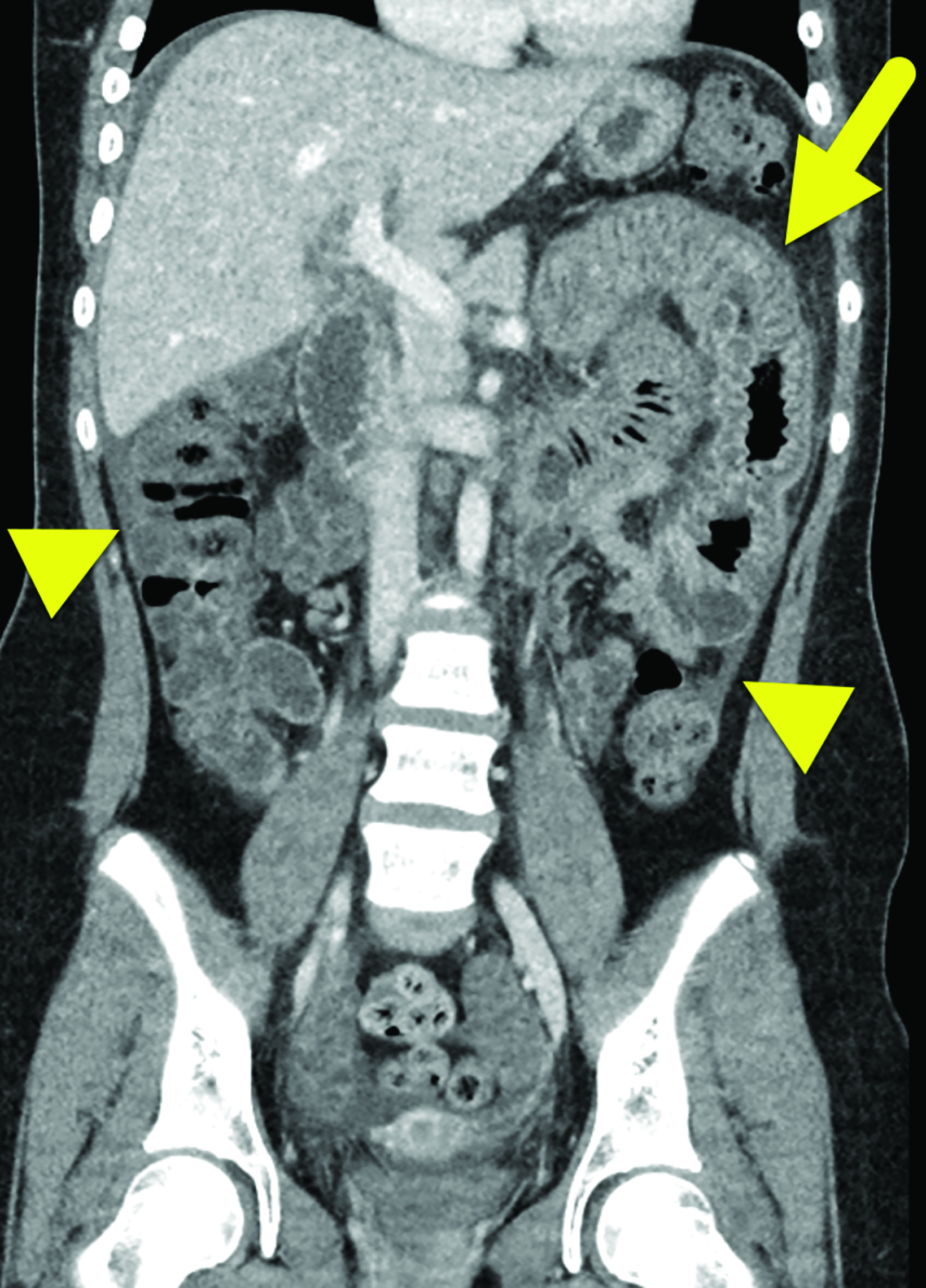
Computed tomography (CT) of the abdomen and pelvis with contrast (Figure 1) showed wall thickening involving the distal duodenum and proximal jejunum. There was contrast enhancement of the mucosa and submucosal edema. The enhancing bowel loops were mildly distended with fluid and there was a small volume of ascites.
Diagnosis
Hereditary angioedema
The differential diagnosis for focal small-bowel wall thickening in an adolescent includes enteritis, Crohn disease, ischemic bowel, and lymphoma.1 In this patient, the diagnosis of HAE was known, making the other entities less likely.
Leukocytosis with neutrophilia is often present during episodes of acute HAE.2 It is important to differentiate bradykinin-mediated angioedema from histamine-mediated angioedema, as the latter has features of urticaria and pruritis and usually involves triggers such as drugs, foods, and viruses.
Discussion
Hereditary angioedema is an autosomal dominant disorder typically resulting from the lack (HAE type 1) or dysfunction (HAE type 2) of C1-inhibitor protein.3 C1-inhibitor protein primarily acts to control the creation of kinin proteins such as bradykinin and to limit the activation of the intrinsic complement cascade.4 Bradykinin is a protein that acts as a potent vasodilator and is known to be the principal mediator of angioedema.
Local trauma or stress may trigger these cascades, resulting in significant angioedema. The classic trigger for HAE is angiotensin-converting enzyme (ACE) inhibitor use because ACE breaks down bradykinin. When patients with genetically impaired C1-inhibitor function use ACE inhibitors, bradykinin levels can markedly increase, leading to angioedema.4 Though ACE-inhibitors are primarily used for hypertension management in adults, they play a role in heart failure management in the pediatric population.5
The estimated prevalence of HAE is 1 in 50,000; it typically presents within the first two decades of life. There tends to be a strong family history of HAE, although spontaneous HAE occurs in up to 25% of patients.6
Typically, symptoms of HAE involve the upper airway, skin, and gastrointestinal (GI) tract. Symptoms include skin swelling that is deforming and painful but not pruritic, upper airway swelling, which can result in dyspnea and even asphyxiation; and GI symptoms including obstruction and pain.4 Attacks of HAE last 2 to 5 days and usually resolve without therapy. Prodromal symptoms, including an erythema marginatum rash, are possible.4
In HAE with GI involvement, contrast-enhanced abdominal CT typically shows bowel wall thickening, often with mural stratification or the “halo” sign, a result of a thickened, low-density submucosal layer secondary to edema with mucosal and subserosal enhancement.6 It is possible for wall thickening to be asymmetric as opposed to circumferential.6 There is often adjacent free fluid.6 Imaging findings are typically nonspecific given the number of other causes of intestinal wall edema.1 The edema is usually segmental, with the duodenum and jejunum most commonly affected during an acute episode of HAE involving the bowel;7 the ileum, colon, and stomach are much less frequently involved.7
Sonography is often utilized in the pediatric population with findings of HAE and may show free intraperitoneal fluid and bowel wall edema.8 Magnetic resonance imaging may be used to confirm bowel wall thickening but is more likely to be used for brain imaging when there is concern for associated cerebral edema in the setting of HAE with GI involvement.10
HAE is confirmed through serum assays, including measurement of C4 complement, concentration of C1-INH, and functional C1-INH.9 The C4 level, a highly sensitive measure, is typically less than 30% of mean normal levels.9 Additional laboratory findings may include a leukocytosis with neutrophilic predominance and varying levels of c-reactive protein from normal to elevated, including elevations at baseline.11
The greatest morbidity from HAE comes from laryngeal edema; thus, management of acute attacks should focus on ensuring airway patency.4,5 Additionally a concentrate of C1-INH may be helpful during acute attacks and as prophylaxis, but its cost and availability limit its use.2,4 In a patient with recurrent episodes of unexplained abdominal pain with no identifiable trigger and CT findings reflecting bowel wall edema, HAE should be considered as a diagnosis.
References
- De Backer AI, De Schepper AM, Vandevenne JE, et al.. CT angioedema of the small bowel. Am J Roentgenol. 2001; 176(3):649-652.
- Ohsawa, I., Nagamachi, S., Suzuki, H. et al. Leukocytosis and high hematocrit levels during abdominal attacks of hereditary angioedema. BMC Gastroenterol. 2013, 123. https://doi.org/10.1186/1471-230X-13-123
- Ciaccia D, Brazer S, Baker M. Acquired C1 esterase inhibitor deficiency causing intestinal angioedema: CT appearance. Am J Roentgenol. 1993;151:1215-1216.
- Caccia S, Suffritti C, Cicardi M. Pathophysiology of hereditary angioedema. Pediatr Allergy Immunol Pulmonol. 2014;27(4):159-163.
- Momma K. ACE inhibitors in pediatric patients with heart failure. Paediatr Drugs. 2006; 8(1):55-69.
- Banday AZ, Kaur A, Jindal AK, Rawat A, Singh S. An update on the genetics and pathogenesis of hereditary angioedema. Genes Dis. 2019;7(1):75-83.
- Ishigami K, Averill SL, Pollard JH, McDonald JM, Sato Y. Radiologic manifestations of angioedema. Insights Imaging. 2014; 5(3):365-374.
- Henao MP, Kraschnewski JL, Kelbel T, Craig TJ. Diagnosis and screening of patients with hereditary angioedema in primary care. Ther Clin Risk Manag. 2016; 12:701-711.
- Farkas H, Harmat G, Kaposi PN, Karádi I, Fekete B, Füst G, Fáy K, Vass A, Varga L. Ultrasonography in the diagnosis and monitoring of ascites in acute abdominal attacks of hereditary angioneurotic oedema. Eur J Gastroenterol Hepatol. 2001; 13(10):1225-1230.
- Ali MA, Borum ML. Hereditary angioedema: what the gastroenterologist needs to know. Clin Exp Gastroenterol. 2014; 7:435-445.
- Hofman ZL, Relan A, Hack CE. C-reactive protein levels in hereditary angioedema. Clin Exp Immunol. 2014 Jul; 177(1):280-286.
References
Citation
LM L, RB T, CM S, AJ T.Hereditary Angioedema. Appl Radiol. 2022; (4):50-51.
July 14, 2022