Germinal Matrix Hemorrhage
Case Summary
A neonate was born prematurely at 33+0 weeks’ gestation. In utero diagnoses inlcuded interrupted aortic arch and suspected tracheoesophageal fistula. A second neonate was born prematurely at 25+0 weeks’ gestation. The infant had a history of spontaneous intestinal perforation.
Imaging Findings
Neonate 1 developed bilateral grade 2 germinal matrix hemorrhage (GMH, Figure 1 ). Neonate 2 developed periventricular hemorrhagic infarctions in the periventricular white matter with associated mass effect and secondary ventricular enlargement ( Figure 2 ).
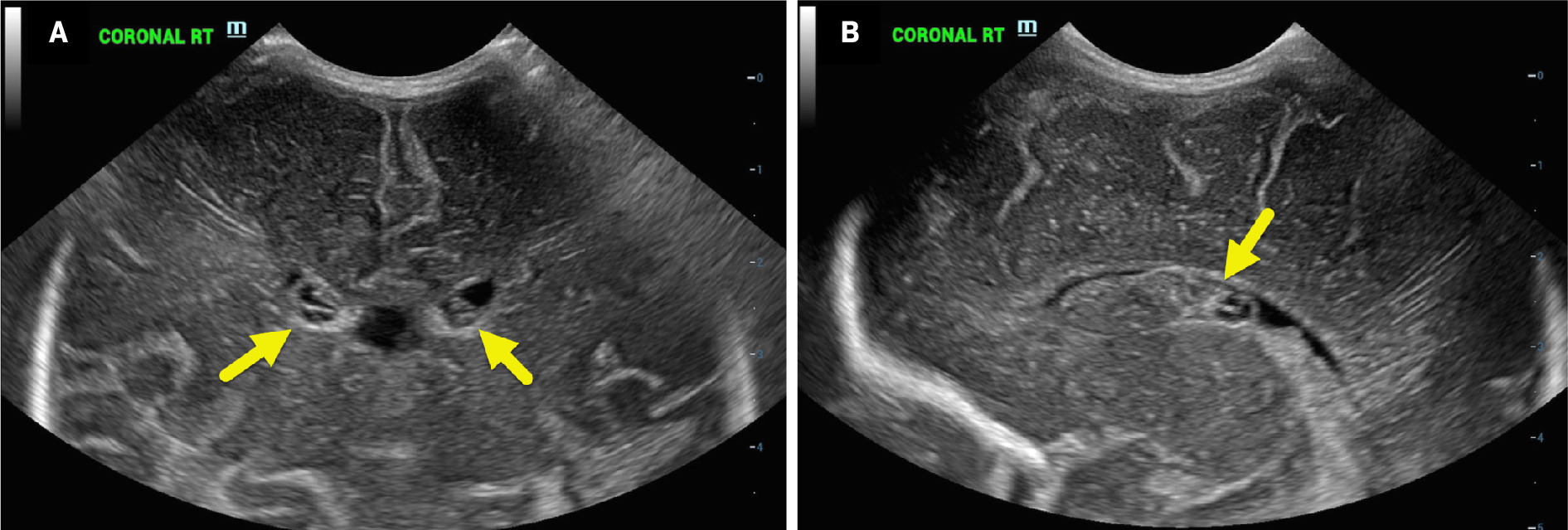
Head US on neonate 1 (born at 33 weeks’ gestation) was obtained at 6 days of life. The coronal image (A) shows bilateral, grade 1 germinal matrix hemorrhages that are primarily hypoechoic (arrows). In a parasagittal plane (B), the one of the hypoechoic hemorrhages is seen in the right caudothalamic groove (arrow).
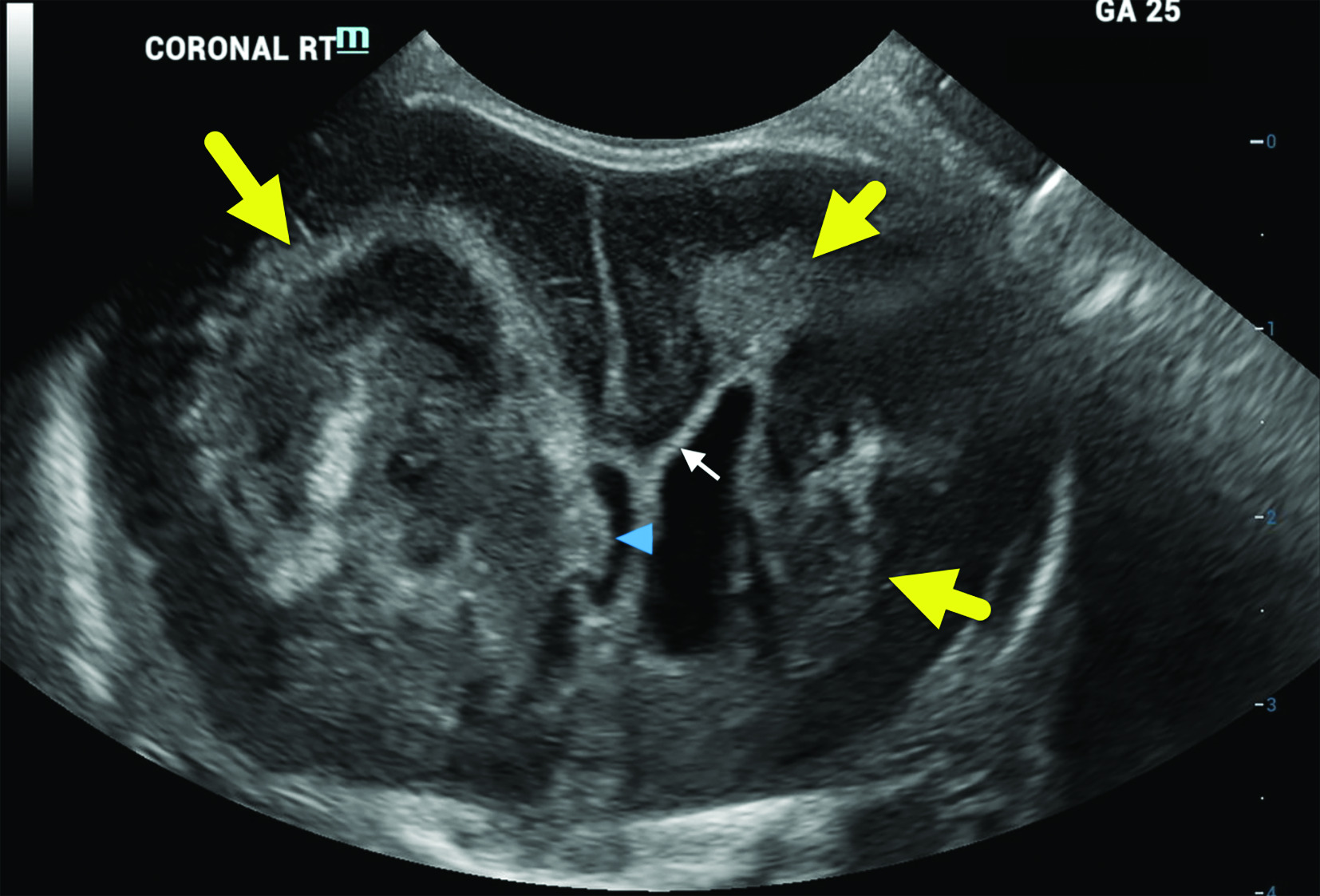
Head US on neonate 2 (born at 25 weeks’ gestation) was obtained at 1 day of life. The coronal view demonstrates massive lateral ventricular enlargement with a large, heterogeneously hyperechoic area involving the right frontal white matter and local mass effect (larger arrow). Part of the blood clot is protruding into the right lateral ventricle (arrowhead). Increased frontal white matter hyperechogenicity representing gliosis or infarction is also seen in the left frontal white matter (shorter arrows).
Diagnosis
Germinal matrix hemorrhage in 2 premature infants (grade 1 GMH in neonate 1 and grade 4 in neonate 2).1
Discussion
GMH is a common complication of preterm infants younger than 37 weeks, especially neonates less than 32 weeks of gestation, and can lead to significant brain injury. Complications of GMH include IVH, periventricular hemorrhagic infarction (PVHI), posthemorrhagic hydrocephalus, cerebellar hemorrhagic injury, and periventricular leukomalacia.1 The outcome depends on the severity of the GMH and any associated brain injury.
The germinal matrix is composed of immature vasculature in the periventricular subependymal region of the developing brain2 and is most prominent around the head of the caudate nucleus. This is the site of mitosis for neuroblasts and glioblasts before their migration out into the brain.3 The germinal matrix reaches maximum volume at 24 weeks’ gestation and slowly decreases until it is fully involuted around 36 weeks’ gestation.4, 5 Owing to the immaturity of the vasculature and the limited connective tissue support, the germinal matrix is at risk of rupture if the infant is born before complete involution.4
Two common grading scales classify severity of GMH: Papile and Volpe. The Papile grading scale was originally published in the 1970s using CT scans and was later modified to include head US findings.6 In this grading scale, 4 tiers classify the hemorrhages from minor (grades 1 and 2) to severe (grades 3 and 4). Grade 1 is defined as hemorrhage contained within the germinal matrix; in grade 2, the hemorrhage extends intraventricularly without ventricular dilatation; in grade 3, ventricular dilatation is noted secondary to the ventricular hemorrhage; and in grade 4, intraparenchymal hemorrhage can be seen.2, 6
The Volpe grading scale also uses tiers of increasing severity of GMH. Volpe grading criteria initially also included grades 1-4; however, grade 4 has been separated into its own distinct category described as PVHI.5 Again, as with Papile, Volpe categories can be grouped as minor hemorrhages (grades 1 and 2) to severe (grade 3 and PVHI). For Volpe, grade 1 is defined as a GMH with little to no IVH seen; in grade 2, the hemorrhage extends into the ventricle, filling 10% to 50% of ventricular volume at the parasagittal section; in grade 3, the IVH fills more than 50% of the ventricular volume, creating ventricular dilatation; and lastly, previously grade 4, is PVHI.5, 6
Up to 50% of neonates with GMH present with no signs.5 - 8 Typically, lower-grade hemorrhages in asymptomatic patients are identified using cranial US, which supports the need for routine screenings in high-risk neonates. Approximately 80% to 90% of GMH-IVHs occur in the first 72 hours of life, while up to 50% of cases occur in the first 24 hours.9 Neonates with severe GMH or PVHI may present with changes in their level of consciousness, bulging fontanelle, decreased movement, abnormal muscle tone, apnea, bradycardia, acidosis, and anemia.2, 7 The more severe cases may progress to respiratory failure, seizures, fixed pupils, decerebrate posturing, coma, and death. Those who survive higher-grade GMH are more likely to develop long-term complications such as cerebral palsy, cognitive delay, sensory deficits, and epilepsy.4, 6
The risk of GMH increases as the gestational age at birth decreases.1, 7 The incidence of severe lesions (grade 3 and PVHI) is 10% to 25% in survivors born at 24 weeks. In contrast, severe injuries are only seen in fewer than 5% of those born after 28 weeks’ gestation and are rarely observed beyond 32 weeks’ gestation.7 Additionally, the overall incidence of GMH is 20% to 25% of very-low-birth-weight infants.7, 8
Head US is the initially recommended screening tool owing to its low cost, portability and bedside use, and lack of ionizing radiation. CT and MRI may also be used; however, CT is uncommonly used. When identifying white matter lesions secondary to GMH, MRI is the gold standard.9 To monitor the appearance or progression of GMH, serial head USs are recommended.7 Although protocols may differ by institution, for neonates born less than 28 weeks’ gestation or 1000 g, it is recommended that scans be performed on days 1, 3, 7, 14, 21, 28, and then every other week until the infant reaches term-equivalent age. If the neonate is born at more than 28 weeks’ gestation, serial cranial USs should be performed on days 1, 3, 7, 14, 28, week 6, and at term-equivalent age.7
The main protective factor in reducing GMH is preventing preterm delivery. When not possible, prenatal glucocorticoids have proven effective, as has generally minimizing trauma and stimulation of the newborn. Decreasing this stimulation includes minimizing post-delivery transport when possible, minimizing suctioning and handling, and optimizing ventilation.4 Maintaining stable cerebral blood flow is crucial, which may include supporting steady blood pressures with intravenous fluids and blood products, as needed. Administration of indomethacin to promote closure of the patent ductus arteriosus may be used in the setting of grade 3 or 4 GMH; however, this may lead to oliguria, decreased kidney perfusion, gastrointestinal bleeding, and necrotizing enterocolitis.6 Additionally, in cases of posthemorrhagic hydrocephalus, intervention may be required, although the efficacy of these interventions is not fully evident.6
Conclusion
GMH is a common complication of prematurity that can have profound consequences and significant long-term effects. Up to 50% of neonates with GMH can initially present with no signs, which is why serial head US screening in preterm infants is necessary. Head US is the primary modality for diagnosis and grading, and can help predict outcomes for the infant to plan potential interventions.
References
Citation
Williams AA, Towbin RB, Schaefer CM, Towbin AJ.Germinal Matrix Hemorrhage. Appl Radiol. 2024; (6):36 - 38.
doi:10.37549/AR-D-24-0013
December 1, 2024