Focused Ultrasound Improves Symptoms, Reduces Plaque in Alzheimer Disease Clinical Trial
Images
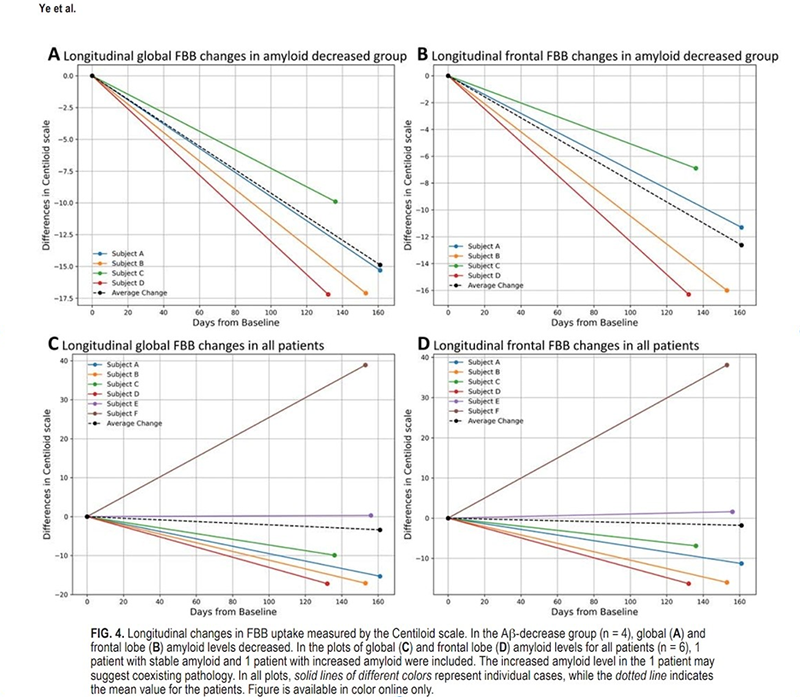
Results from a clinical trial using focused ultrasound to treat Alzheimer disease were published in the Journal of Neurosurgery, representing an important new direction in the treatment of Alzheimer. Led by Jin Woo Chang, MD, PhD, in Seoul, Korea, the study demonstrated that repetitive focused ultrasound–mediated blood-brain barrier (BBB) opening on both frontal lobes is safe and can reduce amyloid plaques – a hallmark of Alzheimer –even without the co-current administration of Alzheimer drugs. The study also found improvement in common neuropsychiatric symptoms associated with the disease.
The clinical trial, funded by the Focused Ultrasound Foundation, evaluated the safety and efficacy of opening larger volumes of the BBB more frequently as compared to prior studies. Each participant – six women aged 50 to 85 – underwent BBB opening in both frontal lobes three times at two-month intervals. The BBB opening treatments were performed using intravenously injected DEFINITY (perflutren lipid microsphere) microbubbles in combination with the ExAblate Neuro 220 kHz low-intensity focused ultrasound system.
The study’s average focused ultrasound–mediated BBB opening volume of 43.1 cubic centimeters is twice as large as Dr Chang’s previous trials and larger than any other previous clinical trial. This achievement exposed more amyloid plaques in the brain to focused ultrasound than ever before, representing an important milestone in Alzheimer treatment.
“We developed this protocol to provide optimal benefit and to test the use of focused ultrasound in larger regions of the brain that are affected by Alzheimer disease,” said Dr Chang, professor of neurosurgery at Korea University Anam Hospital, who collaborated on this study with neurologists from Yonsei University. “This study begins to provide a more complete understanding of the effects of BBB opening alone and will serve as the basis for future trials comparing any potential benefit of adding drug delivery to the affected areas of the brain.”
Amyloid plaque levels and clinical assessments were measured before and after treatment. No medications were administered, allowing researchers to isolate the effects of the focused ultrasound–mediated BBB opening alone.
Improvements in the CGA-NPI score – a clinical test evaluating neuropsychiatric symptoms associated with Alzheimer such as delusions, agitation, irritability, and anxiety – were observed in five of the six participants (83%). No treatment-related adverse events were reported, underscoring the safety of the approach.
“The results of this small study are encouraging, exciting, and provocative but have to be confirmed by larger studies,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. “Alzheimer research has remained relatively stagnant over the past few decades, but focused ultrasound offers hope in a field that has long sought innovative solutions and has the potential to disrupt the course of this devastating disease. Knowing what happens with blood-brain barrier opening in the absence of drugs in patients with Alzheimer disease adds a tremendous amount of knowledge to the field.”
Building on the promising results of this study, the Focused Ultrasound Foundation is funding a subsequent study to investigate the effects on cognition of even larger BBB opening volumes. The trial will also explore the potential benefits of combining focused ultrasound with drug delivery to optimize treatment outcomes for Alzheimer patients.