FDA Breast Density Reporting is in Effect. Ready for Patient Conversations?
As of September 10, 2024, all mammography facilities in the United States must inform a patient after their mammogram if their breasts are “dense” or “not dense”.1 For those with dense breasts, patients must also receive the following information: “Dense tissue makes it both harder to find breast cancer on a mammogram and raises the risk of developing breast cancer. Your breast tissue is dense. In some people with dense tissue, other imaging tests in addition to a mammogram may help find cancers. Talk to your healthcare provider about breast density, risks for breast cancer, and your individual situation.”1 The FDA anticipates these notifications will contribute to reduced breast cancer mortality and lower treatment costs.1
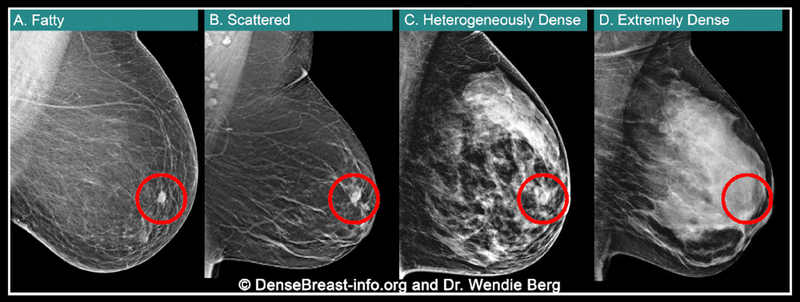
And, the September 2024, updated reporting requirements, for the first time also specify that density reporting to referring providers must include an assessment of breast density classified as one of four categories:1
- “The breasts are almost entirely fatty.”
- “There are scattered areas of fibroglandular density.”
- “The breasts are heterogeneously dense, which may obscure small masses.”
- “The breasts are extremely dense, which lowers the sensitivity of mammography.”
(Note that in July 2025, the FDA also issued alternative phrasing to be used in provider reports of unilateral mammograms.2)
Of the four density categories, the last two (image), heterogeneously dense and extremely dense, are considered “dense breasts.”1 Patients may be surprised to learn the limitations of mammograms in dense breasts; a “normal” or “negative” mammogram report for patients with dense breasts doesn’t reliably mean cancer isn’t present. In fact, in patients with extremely dense breasts, mammograms will miss about 40% of cancers present.
When a patient with dense breasts asks about other imaging tests, are you ready to be a resource?
Health providers should be prepared to answer patient questions about the screening and risk implications of dense tissue and/or provide resources on the topic. Supplemental screening considerations should include assessment of the patient’s breast density, other risk factors, patient preference, and availability of imaging.4 A lifetime risk of at least 20% is considered “high risk” and most current national breast cancer screening guidelines address women at high risk.4 However, even without formal risk assessment, health care providers can identify patients who do meet criteria for supplemental screening. For instance, women with dense breasts and even one other risk factor, like family history, frequently meet high-risk screening guideline criteria with an estimated lifetime risk of at least 20%. Further, both the National Comprehensive Care Network (NCCN) and the European Society of Breast Imaging (EUSOBI) now recommend MRI screening for women with extremely dense breasts and no other risk factors.4,5
Who Needs More Screening?
Guidelines vary by medical society and the DenseBreast-info.org Medical Advisory Board has developed a helpful screening decision support tool, “Who Needs More Screening?”.6 Based on the best available evidence, the flowchart details supplemental screening considerations to optimize cancer detection.
NOTE: This flow chart represents the consensus opinion of our medical reviewers based on available evidence. The proposed strategy is relatively aggressive, designed to optimize cancer detection. Every technology may not be available at every site. Other guidelines may recommend a later start or different screening frequency.
This is not intended to be a substitute for medical advice from a physician or to create a standard of care for health care providers.
Abbreviations used: MRI = magnetic resonance imaging; DBT = digital breast tomosynthesis; PHBC = personal history of breast cancer; dx = diagnosed; XRT = radiation therapy; yr = year; yrs = years 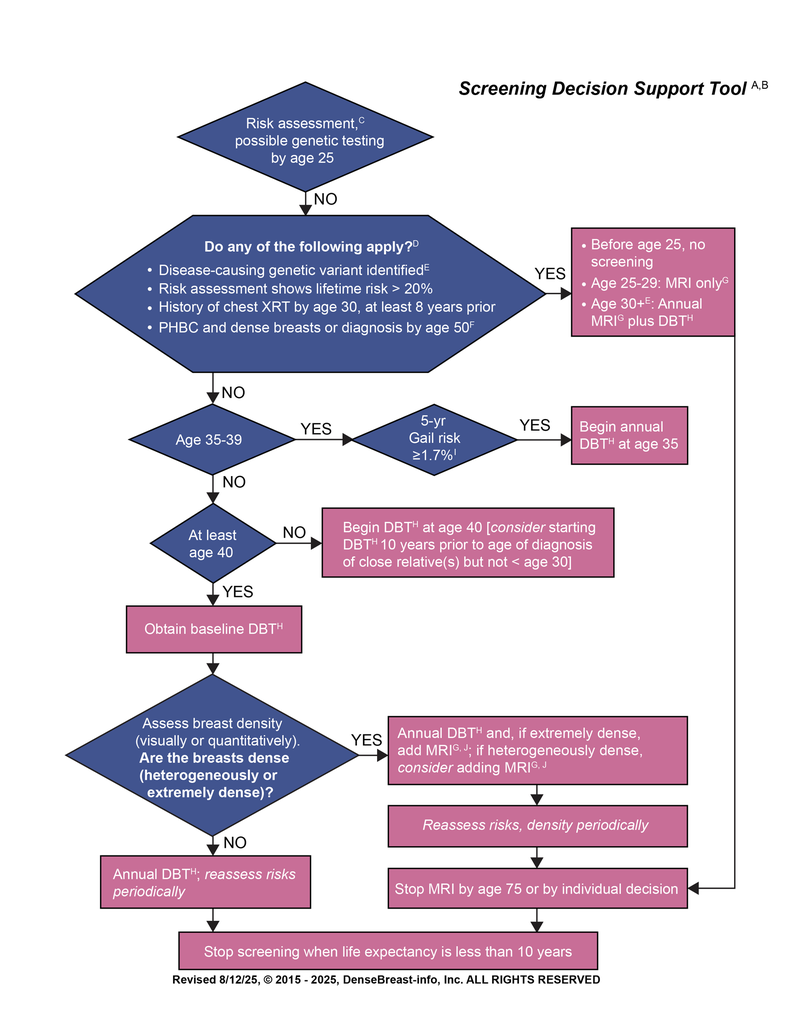
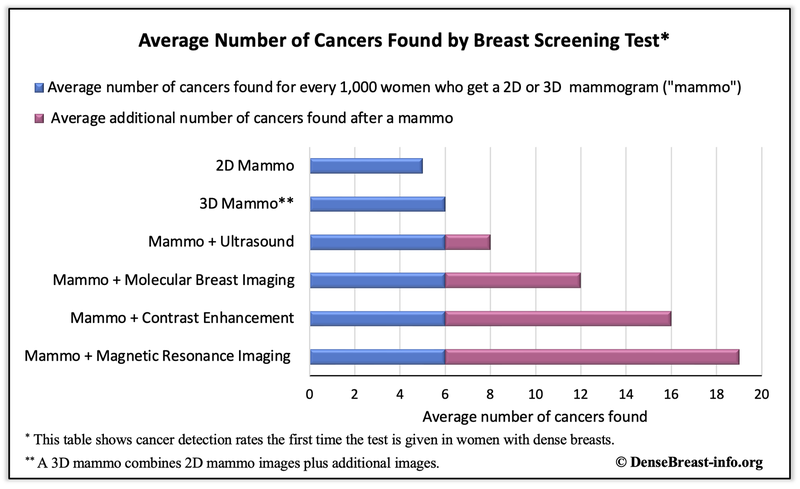
How many additional cancers will be found?
In the table below13, the blue bar shows the average number of additional cancers found for every 1,000 women with dense breasts who get mammography (2D or 3D) and then supplemental imaging. The maroon bar represents the average additional number of cancers found, by modality, if the patient is sent for additional screening after the mammogram. As detailed, adding ultrasound to mammography increases cancer detection by 2 cancers/thousand women screened. Adding MBI to mammography detects an additional 6 cancers/thousand women screened. Adding CEM to mammography detects an additional 10 cancers/thousand women screened but can also replace mammography as the low-energy image mimics a standard mammogram. And MRI detects an additional 13 cancers per thousand women screened.
With the relatively recent FDA mandated dense breast reporting requirements, every health provider involved with a woman’s breast screening should be prepared to answer questions about dense breasts, risk, and supplemental screening.1 To improve the early detection of breast cancer and ensure personalized care, it is suggested that imaging centers implement formal protocols that include evaluation of patient risk factors (including breast density), patient need for supplemental imaging and best practices for communication with referring providers to facilitate imaging orders. And, still needed is a federal insurance mandate, like the Find It Early Act described above, that would help provide “imaging equity” to all patients by removing potential financial barriers to supplemental imaging. Until we can prevent breast cancer entirely, finding it early — when most treatable and survivable, is an alternative we should all strive for.
Footnotes
- Modified from Berg WA, Rafferty EA, Friedewald SM, Hruska CB, Rahbar H. Screening Algorithms in Dense Breasts: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2021;216(2):275-294 and National Comprehensive Cancer Network. NCCN Guidelines. Breast cancer screening and diagnosis v.2.2025, March 20, 2025
- For cancer detection by modality, see DenseBreast-info.org (Technology Tab/Table: Summary of Cancer Detection by Screening Method).
- Using risk models largely dependent on family history, such as the Tyrer Cuzick (IBIS), BOADICEA (CanRisk), or BRCAPRO models, but NOT the Gail model; see DenseBreast-lnfo.org (Health Professional Tab/Risk Models).
- Women with LCIS (lobular carcinoma in situ) or ADH (atypical ductal hyperplasia) should consider supplemental surveillance with MRI, especially if other risk factors are present.
- Age to start screening MRI varies by genetic variant. See Risk Models Table (Health Professional Tab/Risk Models).
- Others with PHBC should strongly consider supplemental screening with MRI, especially if other risk factors are present.
- For those who qualify for but cannot have MRI, consider contrast-enhanced mammography (CEM) or molecular breast imaging (MBI) if over age 30. If not possible, consider screening ultrasound (US). For women age 25-29 who meet criteria for screening MRI but who are unable to have it, including pregnant women (any age), consider screening US.
- If DBT is not available, use 2D full-field digital mammography.
- https://bcrisktool.cancer.gov/
- No frequency is specified.
References Cited
- Food and Drug Administration, Mammography Quality Standards Act, Federal Register Vol. 88, No. 47, 2023
- https://www.fda.gov/radiation-emitting-products/regulations-mqsa/mqsa-alternative-standard-26-issuing-report-breast-density-assessment-phrased-singular-or-neither Accessed 12 September 2025
- Destounis S, Johnston L, Highnam R, Arieno A , Morgan R, Chan A. Using Volumetric Breast Density to Quantify the Potential Masking Risk of Mammographic Density, AJR Am J Roentgenol 2017;208(1):222-227.
- Berg WA, Seitzman RL, Pushkin J. Implementing the National Dense Breast Reporting Standard, Expanding Supplemental Screening Using Current Guidelines, and the Proposed Find It Early Act. Journal of Breast Imaging, 2023, 712–723.
- NCCN guidelines for patients®, Breast Cancer Screening and Diagnosis, 2025.
- https://densebreast-info.org/for-providers/who-needs-more-screening/
- Monticciolo DL, Newell MS, Moy L, Lee CS, Destounis S. Breast Cancer Screening for Women at Higher-Than-Average Risk: Updated Recommendations From the ACR. J Am Coll Radiol 2023;20:902-914.
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Breast Cancer Screening and Diagnosis, Version 2.2004.
- Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of Abbreviated Breast MRI vs Digital Breast Tomosynthesis for Breast Cancer Detection Among Women With Dense Breasts Undergoing Screening. JAMA. 2020; 323(12):746-756.
- Sorin V, Yagil Y, Yosepovich A, et al. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol. 2018; 211(5):W267–W274.
- https://densebreast-info.org/legislative-information/state-law-insurance-map/
- https://www.congress.gov/bill/118th-congress/house-bill/3086/all-info
- https://densebreast-info.org/screening-technologies/for-patients-screening-tests-after-a-mammogram/
©2025 BAYER, the Bayer Cross, are trademarks owned by and/or registered to Bayer in the U.S. and/or other countries. Bayer HealthCare LLC 100 Bayer Boulevard, PO Box 915, Whippany, NJ 07981
PP-PF-RAD-US-1351-1 September 2025
Citation
FDA Breast Density Reporting is in Effect. Ready for Patient Conversations?. Appl Radiol.
September 26, 2025