Evaluating rheumatoid arthritis with PET
Images
Positron emission tomography (PET) imaging can help clinicians to more fully evaluate the extent of rheumatoid arthritis (RA) by targeting translocator protein (TSPO) expression in joint lining tissue, according to a study published in the July issue of the Journal of Nuclear Medicine. PET radioligands that target TSPO, which is highly expressed on activated macrophages, the immune cells responding to inflammation, have proved an excellent tool for imaging joint inflammation in RA, according to a multi-institutional team of British researchers.
The team sought to investigate TSPO expression in major cellular constituents of RA pannus-monocytes, macrophages, fibroblastlike synoviocytes (FLS cells), and CD4-positive (CD4+) T lymphocytes (T cells)-to more accurately interpret TSPO PET signal from RA synovium. It is the first ever analysis of TSPO expression in the major constituents of RA pannus (inflamed synovium), according to the researchers. The study demonstrated that TSPO PET likely acts as an imaging tool not only of macrophages, but also of activated synovial fibroblasts, a cell group increasingly recognized to play a critical role in RA inflammation.
The study included three RA patients and three healthy volunteers who underwent PET scans of both knees using the TSPO radioligand carbon-11 (11C)-PBR28. In addition, cellular expression of TSPO was examined in synovial tissue from these individuals, plus three more RA patients and three more healthy patients (undergoing knee arthroscopy for injuries). TSPO mRNA expression and hydrogen-3 (3H)-PBR28 radioligand binding were assessed using in vitro monocytes, macrophages, fibroblast-like synoviocytes (FLS) and CD4+ T-lymphocytes.

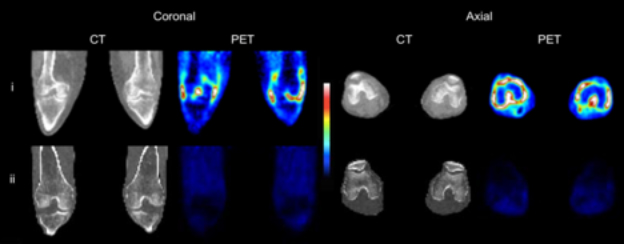
CT and 11C-PBR28 PET SUV images of both knees of (i) an RA patient with clinical signs of synovitis both knees, and (ii) a healthy control.
The11C-PBR28 PET signal was significantly higher in RA joints compared to healthy joints. In addition,3H-PBR28-specific binding in synovial tissue was approximately ten-fold higher in RA patients compared to healthy controls. Immunofluorescence revealed TSPO expression on macrophages, FLS and CD4+ T cells. In vitro study demonstrated highest TSPO mRNA expression and3H-PBR28 specific binding in activated FLS, non-activated and activated ‘M2’ reparative macrophages. The lowest TSPO expression was in activated and non-activated CD4+ T lymphocytes.
Lead author Nehal Narayan, MD, an honorary clinical research fellow in rheumatology of the Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences of the University of Oxford, said in a Society of Nuclear Medicine and Molecular Imaging press release that “TSPO-targeted imaging has long been used as a means of imaging macrophage infiltration in vivo. Numerous studies have demonstrated TSPO-targeted PET as a highly sensitive and specific means of imaging synovitis [inflammation of joint lining tissue], purportedly through imaging synovial macrophage infiltration, a critical process in RA pathogenesis. However, this premise does not take into account the ubiquitous expression of TSPO.”
“It is well recognized that not all currently available treatments are capable of controlling joint inflammation in all patients with rheumatoid arthritis, hence the need to develop new pharmacological therapies,” she said. “This work demonstrates that TSPO PET is able to act as a means of imaging not only synovial macrophages, but also activated synovial fibroblasts. The crucial role of the fibroblast and its soluble products in RA pathogenesis is increasingly realized.”
Noting the recent interest in targeting activated fibroblasts as a novel targeted treatment strategy for RA, Dr. Narayan observed that “TSPO PET imaging in early phase clinical trials may provide a sensitive indication of treatment response to such novel therapies with a view to informing the design of later stage clinical trials. As our knowledge of cellular TSPO expression and behavior grows, TSPO-targeted imaging may also give us unique insights into the pathogenesis of inflammatory disease.”
REFERENCE
- Narayan N, Owen DR, Mandhair H, et al. Translocator protein as an imaging marker of macrophage and stromal activation in rheumatoid arthritis pannus. J Nucl Med. 2018;59(7):1125-1132.