Enlitic Curie Platform Nets FDA Clearance, CE Marking
 The Enlitic Curie platform and the AI application within Curie, Curie|ENDEX, have received regulatory approvals in the US and European Union for commercialization.
The Enlitic Curie platform and the AI application within Curie, Curie|ENDEX, have received regulatory approvals in the US and European Union for commercialization.
"With this commercial approval, we can service the needs across the United States and the European Union,” said Jim Conyers, CEO of Enlitic. “It is critical that we continue to ensure that our products meet and exceed the latest regulatory guidelines so we can do our part in improving the continuum of care. Our team has been hard at work and this approval is a testament to our quest for excellence.”
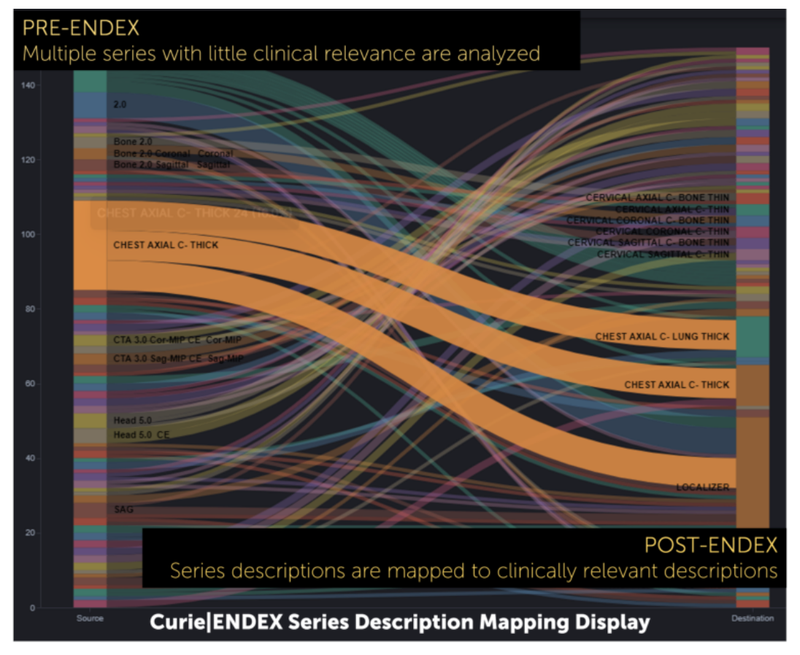
Curie|ENDEX transforms imaging data to a standardized nomenclature and enables relevant clinical content linkage across disparate systems. Doing so gives systems the ability to apply consistent hanging protocols and improve image routing and AI orchestration. In 2020, The University of Texas Health San Antonio (UTHSA) partnered with Enlitic to solve the problem of inconsistent hanging protocols. By standardizing their image data, UTHSA saved an estimated $1 million dollars in time reclaimed over a three-year period.