DSC MRI Perfusion Distinguishes Tumor from Radiation Necrosis
The retrospective study, led by Dr. Michael Iv, MD, Clinical Associate Professor at Stanford University, used Imaging Biometrics, LLC (IB) DSC-MRI software and other advanced imaging methods to evaluate metastatic brain tumors previously treated with a very precise form of radiation therapy called stereotactic radiosurgery. Those other methods included dynamic contrast enhanced (DCE) imaging, arterial spin labeling (ASL) which is an MRI technique that uses the body’s own blood as a contrast agent to compute blood flow measurements, and positron emission tomography (PET) which can detect metabolic differences in tissue.
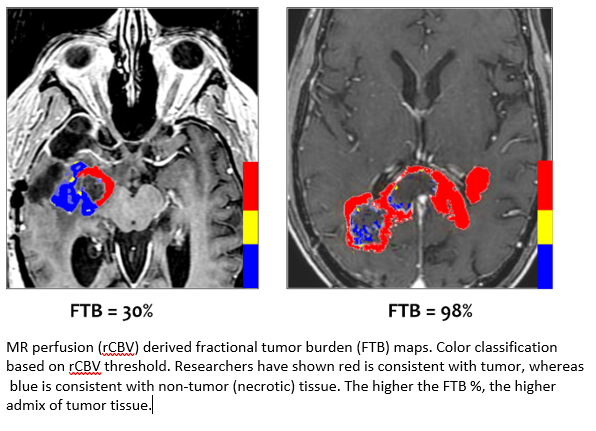
DSC-MRI is well established as the most robust and clinically adopted method for response assessment in high-grade (HG) primary brain tumors. Numerous studies have demonstrated the accuracy and reproducibility of IB Neuro’s DSC platform and, specifically, its standardized measurement of relative cerebral blood volume (rCBV) to distinguish tumor from non-tumor tissue in HG tumors. This study is the first to replicate this method in metastatic brain tumors. The results are positive news for treatment teams who currently must rely on contrast-enhanced MRI to answer the basic question - is the enhancing region tumor, or is it an effect due to treatment?
Metastatic brain tumors are ten times more prevalent than primary brain tumors. This year in the US alone, it is expected that 220,000 people will be diagnosed with a metastatic brain cancer. Moreover, advances in treatment therapy are helping metastatic brain tumor patients live longer with improved quality of life. Thus, this number is expected to increase over time. Up to 50% of all cancers will eventually metastasize to the brain.
The study referred to IB’s standardized rCBV (sRCBV) measurement. Standardization is an exclusive calibration technology that is built-in to IB Neuro. Fully automated, standardization translates the relative MRI image intensities to a fixed and consistent quantitative scale. This machine-learned technology is applied once for all scanner platforms, field strengths, and patients, and enables direct quantitative comparison between scans. It has been shown superior over other tissue normalization techniques; all of which require manual user input and are, therefore, less consistent and cannot be automated. The ability to obtain quantitative sRCBV values on a per-voxel basis differentiates IB Neuro from all other DSC-MRI platforms.
“This relevant and powerful study further validates our decision to develop IB Trax. Incorporating our quantitative Delta T1 (patent-pending) and sRCBV maps will help neuroradiologists track and monitor brain metastases over time and potentially boost their accuracy and efficiency,” said Trevor Brown, CEO and Chairman of IQ-AI, Ltd.
Related Articles
Citation
DSC MRI Perfusion Distinguishes Tumor from Radiation Necrosis. Appl Radiol.
May 4, 2022