Deep Learning: Promising to Revolutionize Image Reconstruction
Images
Artificial intelligence-based image reconstruction tools are poised to revolutionize computed tomography (CT) and magnetic resonance (MR) procedures in 2021. Lower radiation dose, improved image quality, higher scanning efficiency, and positive impacts on workflow and patient experience are among the broad range of benefits deep learning is expected to deliver in the coming year.
Reconstruction Techniques
Traditional MR reconstruction techniques can be limited by long scan duration, artifacts, and signal-to-noise ratio (SNR) constraints. Filtered back projection, the traditional fast CT reconstruction technique, is challenged by mathematical assumptions that manifest as image noise, which can only be overcome by higher radiation doses. Iterative reconstruction (IR) techniques, long popular in CT but also recently becoming available in MR, effectively reduce noise while allowing for optimization of CT dose or MR scan times; however, these techniques may lead to changes in image texture and losses in quantitative integrity.
Enter deep learning reconstruction (DLR) tools, based on a subset of machine learning leveraging neural networks. The myriad benefits of DLR include an even greater boost in SNR than can be delivered by IR, while preserving image texture (noise power spectrum) and quantitative integrity for measures such as CT Hounsfield units and MR anisotropy values.
Strategies To Take Advantage of DLR
Deep learning reconstruction is applied to accelerated or dose-reduced acquisitions to maintain or enhance image quality. With CT, an imaging factor such as tube current or potential is decreased proportionally once the noise reducing capability of the reconstruction is calibrated. For MR, the opportunities for acceleration are varied and complex but based on techniques that trade signal (which DLR can restore) for scan duration.
MR Scan Acceleration
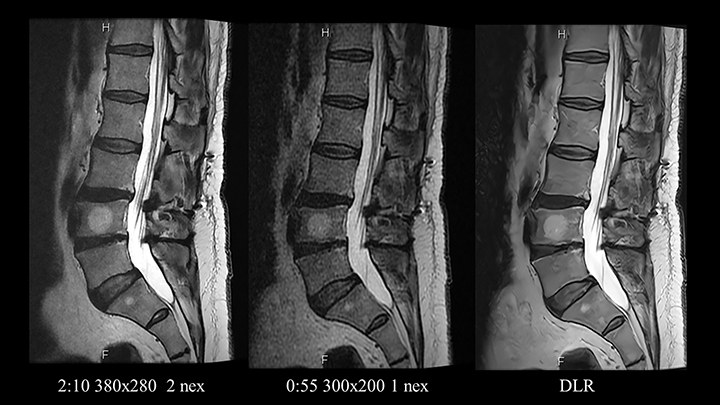
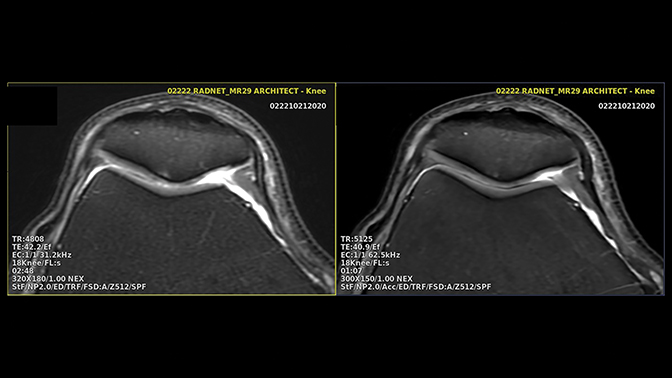
Speeding MR imaging by reducing the acquired matrix will improve SNR and contrast-to-noise ratio (CNR) at the cost of image sharpness. To accelerate imaging without reducing spatial resolution, the first step is to reduce the number of scan averages. On modern machines with high channel count coils this might present an opportunity to accelerate by 30-60% (Figure 1). Older devices, which typically employ a larger number of averages, offer even greater relative acceleration possibilities.
Limits do apply; although scans acquired with a single average are faster, they are susceptible to reconstruction artifacts and are paradoxically more prone to motion. Therefore, two averages may be required for some regions of anatomy and some sequences.
With Cartesian scanning, fractional fields-of-view (FOV) can reduce scan duration, and DLR can restore the proportionally lost SNR. Undersampling techniques, like compressed sensing, can accelerate scans by an incremental 30-50% at the cost of increased image noise. Depending on the original equipment manufacturer (OEM) proprietary implementation, acceleration can be pushed just so far before blurring and other complex losses in image integrity occur. Diving deeper into MR physics reveals additional opportunities; eg, by increasing bandwidth. While bandwidth changes have an inverse square root relationship to signal, increases can improve sharpness (below) and reduce acquisition time by improving slice efficiency.
DL Quality Enhancement
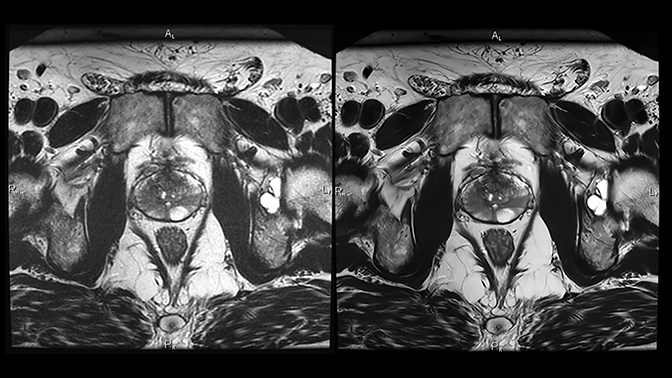
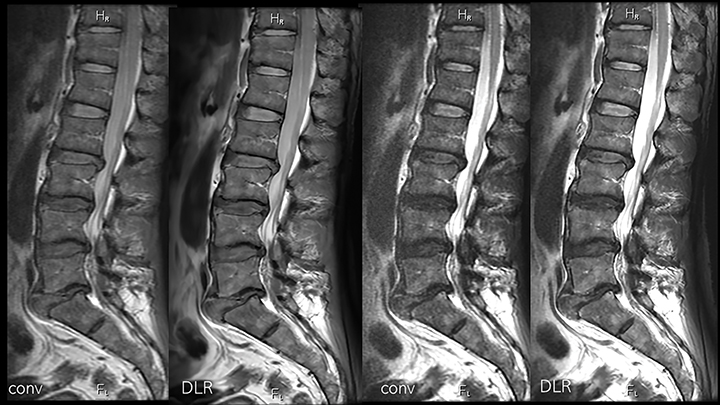
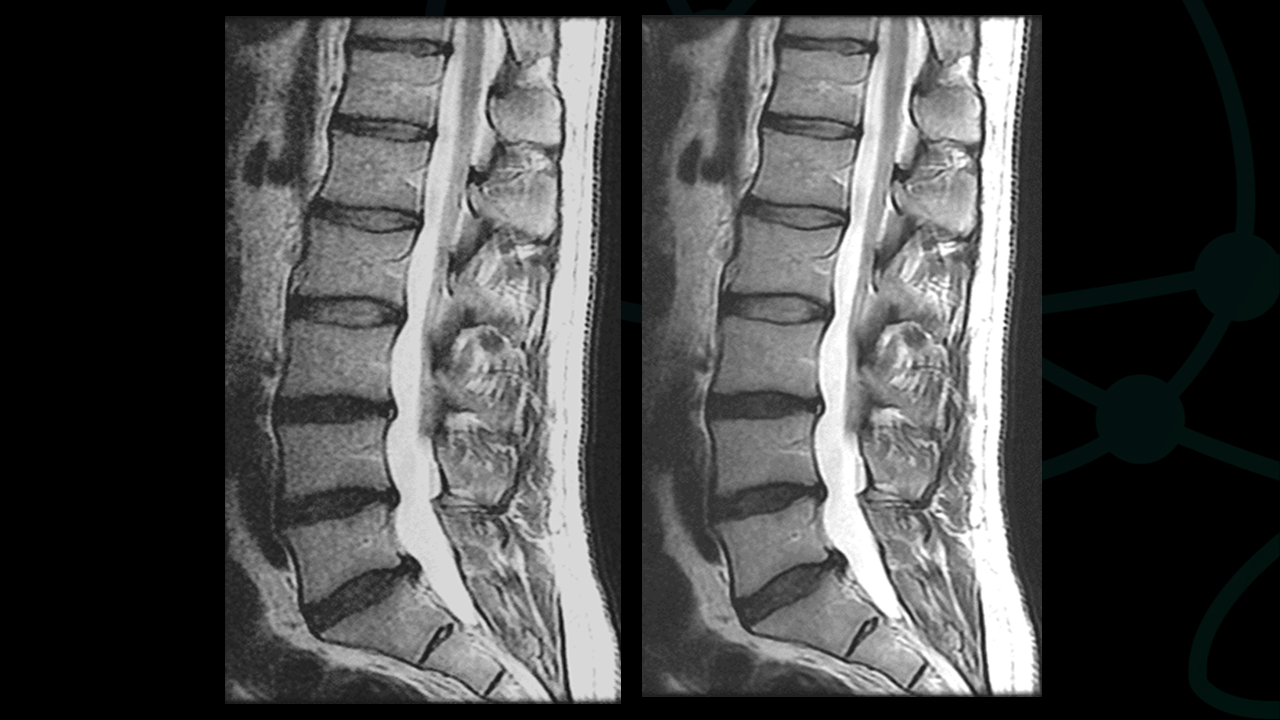
Deep learning reconstruction can actually improve image quality in several ways. (Figure 2). For example, some of the acceleration allowed by DLR can be traded to make the time required for higher imaging matrices practical for routine use (Figure 3). Noise reduction enhances the SNR and CNR of existing acquisitions, sometimes enabling use of a smaller FOV than was previously feasible. As a result, resolution increases without requiring corresponding phase or frequency matrix increases.
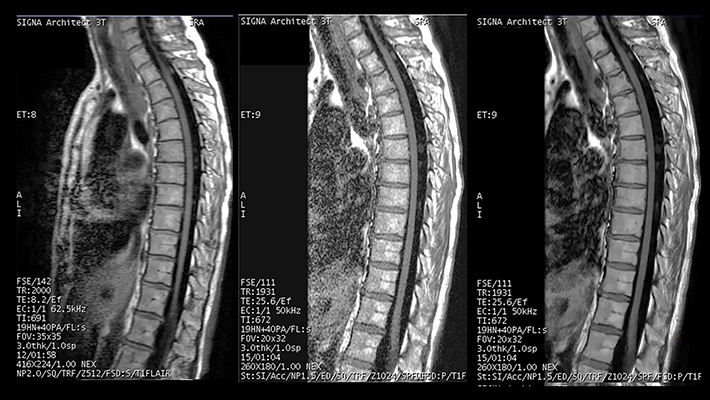
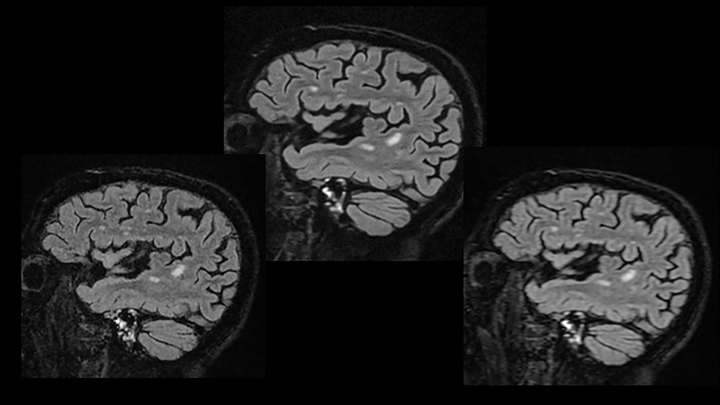
The inherent sharpening properties of DLR can also improve image resolution (Figure 4). Its potent noise reduction facilitates the use of higher bandwidth values yielding lower echo spacing, which reduces blur and increases sharpness (Figure 5). Alternatively, one can opt for speed by balancing the increase in resolution with a lower scanning matrix.
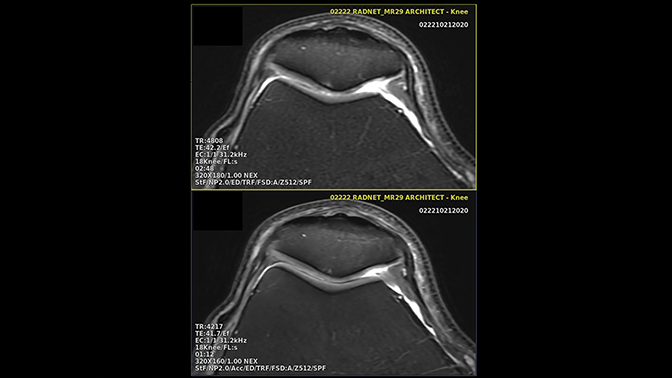
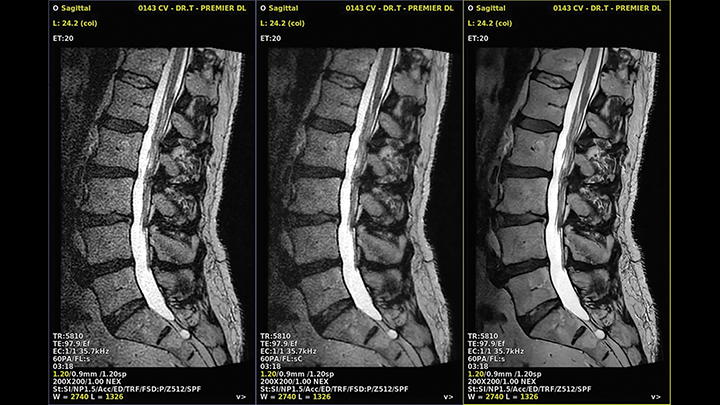
Deep learning reconstruction models can be trained to recognize and eliminate artifacts such as truncation, obviating the need for compensatory apodization filters and their associated trade-off in spatial resolution (Figure 6). Some DLR models offer super-resolution capabilities, wherein the model, trained on high-resolution data, imprints the sharpness on a faster, low-resolution series, coupling the transferred spatial resolution with the higher SNR and CNR of the larger native voxel size series (Figure 7). Deep learning reconstruction is also capable of powerfully denoising a highly signal-starved, ultra-high-resolution image set (Figure 8).
DLR Validation
Qualitatively and quantitatively validating the reliability and generalizability of DLR is important. Without a radiologist’s qualitative assessment of non-inferiority, a reconstruction solution may not be acceptable for clinical use. Recent studies have demonstrated that, despite its 40-60% faster scanning duration, DLR can match or exceed the perceived SNR and spatial resolution of a standard-of-care (SOC) image while maintaining clinical and quantitative integrity.1,2
Processed images should also satisfy quantitative measures to ensure that diagnostically relevant features are not masked or altered, and integrity of the processed image information is maintained. This can be accomplished for some types of models by evaluating the structural similarity index measure (SSIM) of the processed and unprocessed images. The SSIM assesses for the presence or absence of absolute errors (such as anatomic data loss or exaggeration). A recent blinded, multicenter, multireader prospective study confirmed the consistency of the SSIM across the DLR processed and unprocessed accelerated data sets.1
Alternatively, when volumetric quantitative imaging is involved, biomarker consistency can be compared. A multicenter prospective study recently submitted for publication has confirmed the quantitative accuracy of 60% accelerated, DL-processed brain MR image data sets, as demonstrated by high biomarker consistency and matched clinical disease status predictability, while delivering perceived superior image quality compared with the longer SOC exams.2 Quantitative biomarkers such as hippocampal volume, hippocampal occupancy score, superior lateral ventricular volume, and inferior lateral ventricular volume were statistically equivalent for the DL-processed fast scans and the SOC.2
Patient Experience
Magnetic resonance imaging is a stressful experience associated with frank anxiety reactions in up to 30% of patients.3 Motion is a significant challenge in MRI, occurring in 29% of inpatient/emergency department exams and 7.5% of outpatient studies.4 Almost 20% of MRI sequences, moreover, must be repeated due to motion artifact, resulting in significant annual revenue losses per scanner.4 DL-based reconstruction solutions promise shorter exams with less patient motion and greater patient comfort.
Progress in DLR Product Development
Deep learning reconstruction products are increasingly being recognized for their positive impact on scanning efficiency, patient comfort, and image quality. Major scanner OEMs and smaller companies alike are developing and refining DLR solutions.
GE’s TrueFidelity, Canon’s Advanced Intelligent Clear-IQ Engine (AiCE), and Algomedica’s PixelShine all have FDA clearance for applications in CT. GE’s AIRTM Recon DL and Canon’s AiCE have FDA clearance for MR applications. Siemens and Philips are also working toward bringing MR image reconstruction technologies to market. The OEM products are vendor proprietary and more than likely applicable only to their recent models.
The first DL-enhanced MR product to be cleared for marketing by the FDA, SubtleMRTM (Subtle Medical, Palo Alto, CA), is a vendor-agnostic, DICOM-based solution (Figure 9). The advantage of Subtle Medical’s products is their applicability to scanners of any age and manufacturer, which is particularly appealing to imaging enterprises with an extensive and mixed fleet of scanners. SubtleMRTM allows older magnets to undergo a “virtual upgrade” and subsequently deliver image quality and efficiency that match or exceed those of newer scanners.
In summary, DL-enhanced image reconstruction offers imaging enterprises, radiologists, and patients a broad range of benefits, including lower radiation dose, improved image quality, higher scanning efficiency, and positive impacts on workflow and patient experience.
As a result, the technology’s widespread incorporation into radiology practices around the world appears imminent as its list of applications in CT and MR imaging continues to grow.
References
- Bash S, Johnson B, Gibbs W, et al. Deep learning reconstruction enables 40% faster spine MR scans which match or exceed quality of standard of care — A prospective, multicenter, multireader trial. Manuscript submitted for publication, December 8, 2020.
- Bash S, Wang L, Airriess C, et al. Deep learning enables 60% accelerated volumetric brain MRI while preserving quantitative performance – A prospective, multicenter, multireader trial. Manuscript submitted for publication, October 25, 2020.
- Melendez JC, McCrank E. Anxiety-related reactions associated with magnetic resonance imaging examinations. JAMA. 1993;270:745–747. DOI: 10.1001/jama.1993.03510060091039
- Andre J, Bresnahan B, Mossa-Basha M, et al. Toward quantifying the prevalence, severity, and cost associated with patient motion during clinical MR examinations. JACR. 2015;12:689-695. DOI:10.1016/j.jacr.2015.03.007
Citation
S B, LN T.Deep Learning: Promising to Revolutionize Image Reconstruction. Appl Radiol. 2021; (1):32-37.
January 19, 2021