Cysts with masses and masses with cysts: An imaging review of cystic breast masses
Images
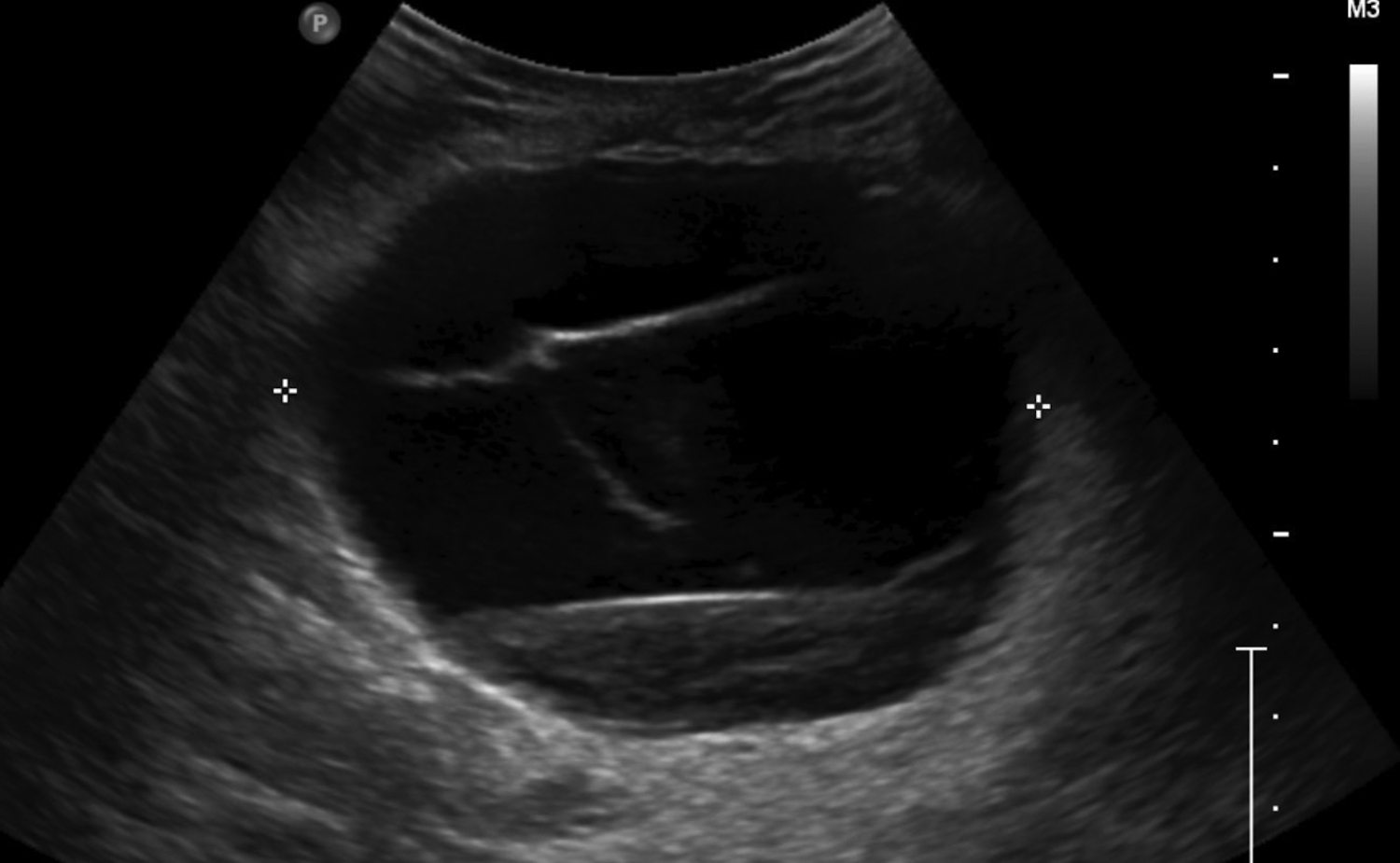
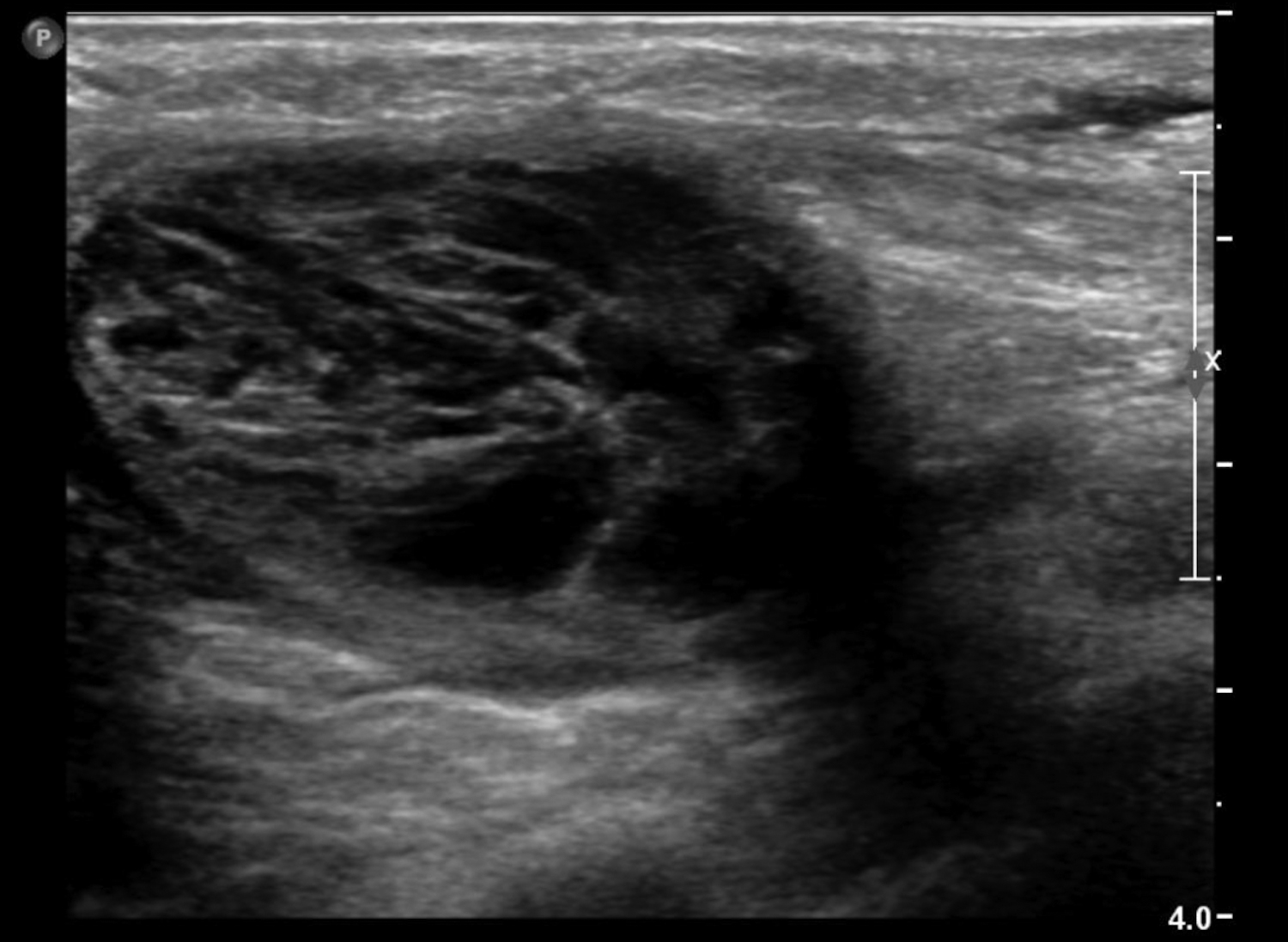

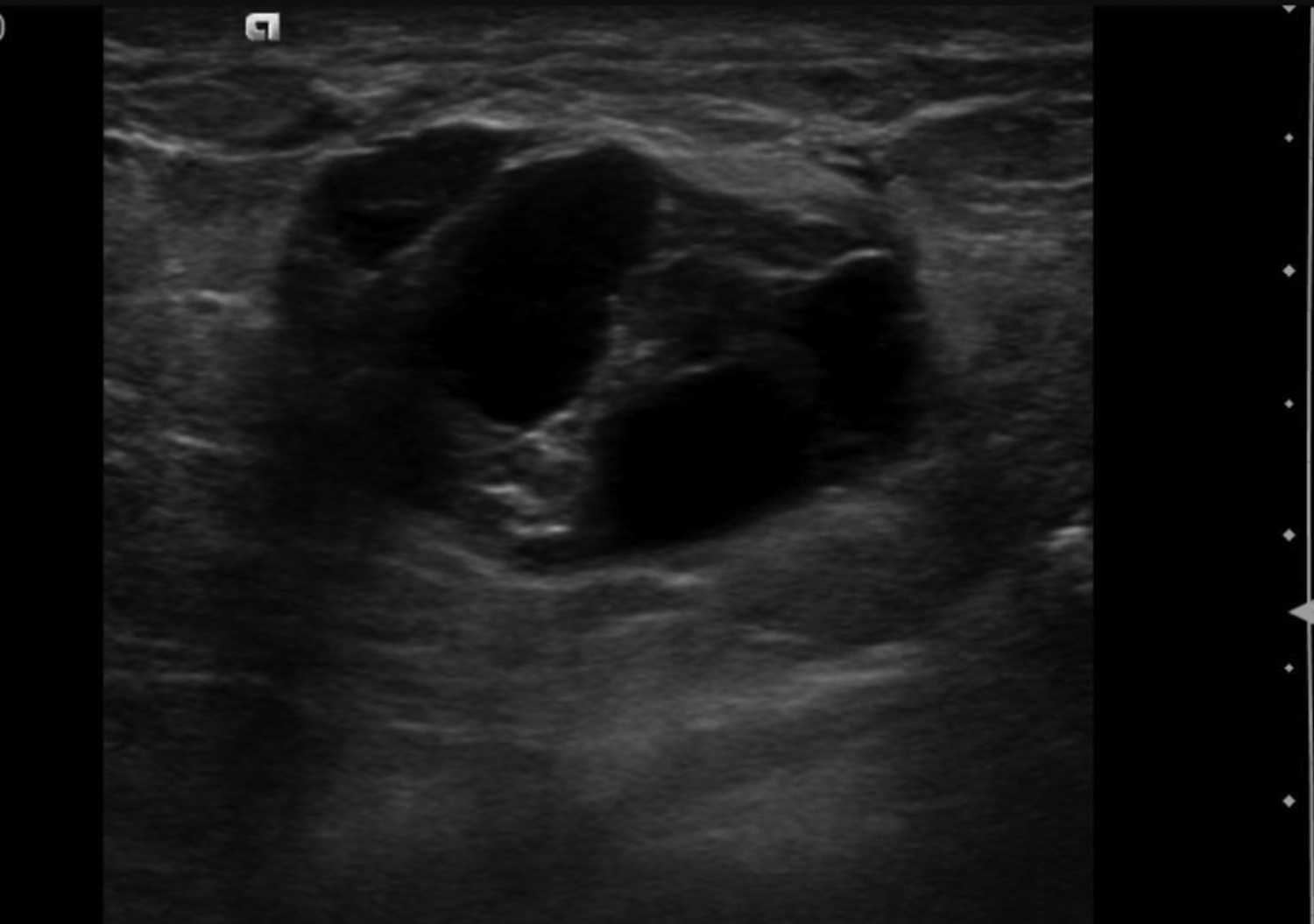
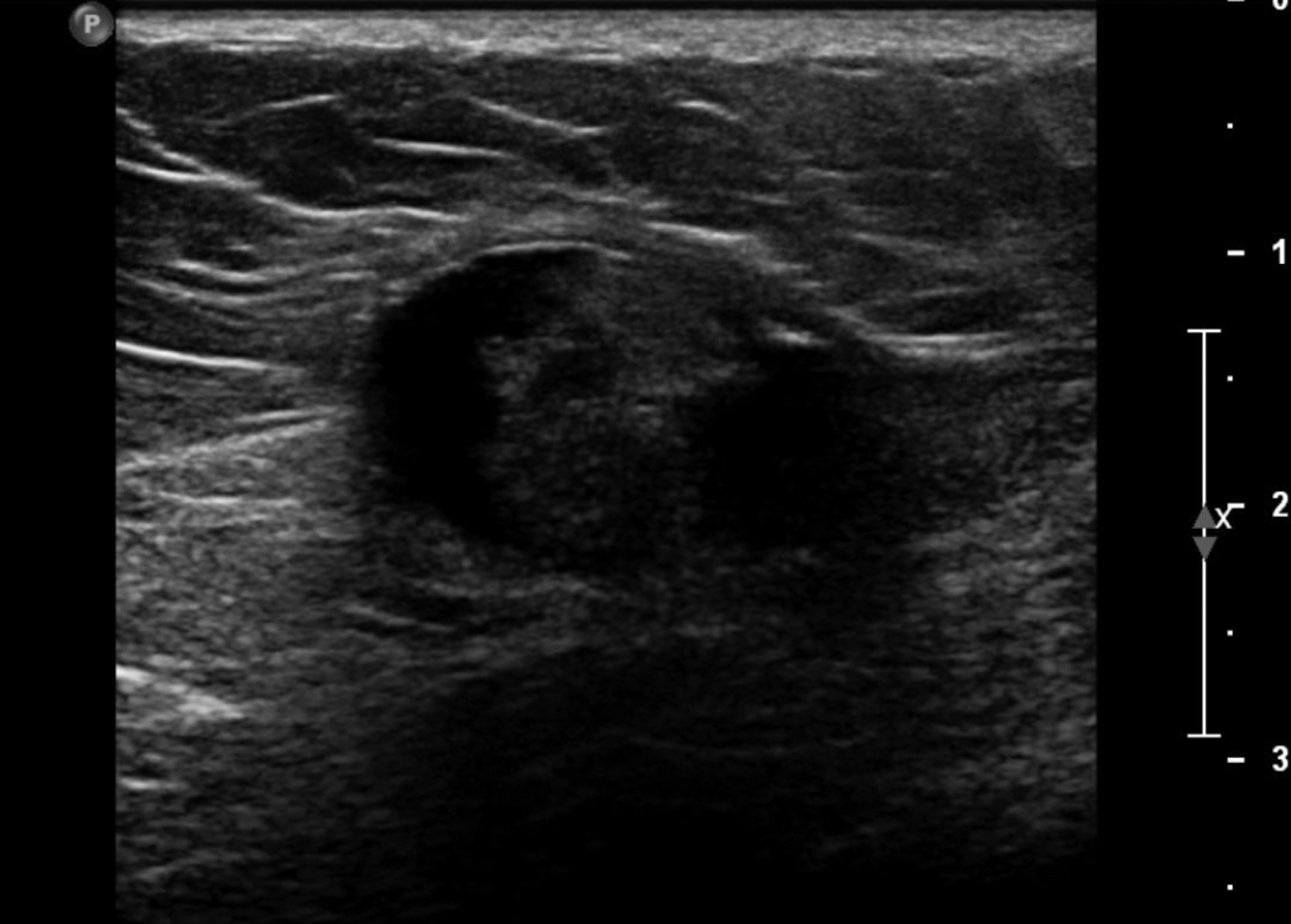








Cystic lesions in the breast commonly present in women aged 30-50 years. They typically appear as circumscribed masses on mammography, but they can be more accurately evaluated on ultrasound.1-2 Assessment of masses on ultrasound is guided by the Breast Imaging Reporting and Data System (BI-RADS) and evaluation includes shape, margin, orientation, echotexture, posterior acoustic changes and vascularity.3 Simple, complicated and clustered cysts are typically benign; however, complex cystic masses containing mixed cystic and solid components are indeterminate. Complex breast cysts have thick septations, thick walls, intracystic masses or other solid components. Between 23% and 31% are associated with malignancy; therefore, biopsy is required.2
Clinical history often narrows the differential diagnosis; however, ultrasound-guided biopsy may be required for definitive diagnosis. Additionally, solid masses may present with cystic spaces, suggesting a different pathology. Given the variable and challenging imaging appearance, we provide this review of the imaging and differential diagnosis for cystic breast lesions.
Benign cystic breast lesions
Simple, complicated and clustered cysts
Simple and complicated cysts are the most common cystic breast lesions. Cysts are fluid filled and develop secondary to dilatation of the terminal ductal lobular unit (TDLU). They are commonly multiple, bilateral and may wax and wane in size. Cysts typically present as circumscribed masses on mammography that maybe obscured by overlying breast tissue.1 Sonographically, simple cysts are circumscribed anechoic masses with posterior acoustic enhancement and absent vascularity. Simple cysts are benign, requiring no further assessment unless aspiration is requested due to symptoms. Complicated cysts contain internal echoes and are associated with less than 2% incidence of malignancy.1-2 Clustered microcysts are multiple grouped cysts and in some instances may require aspiration to distinguish them from solid masses. Follow up, however, is supported by the Berg et al study, where none of the simple, complicated or clustered cysts were found to be malignant.4
Fluid collections
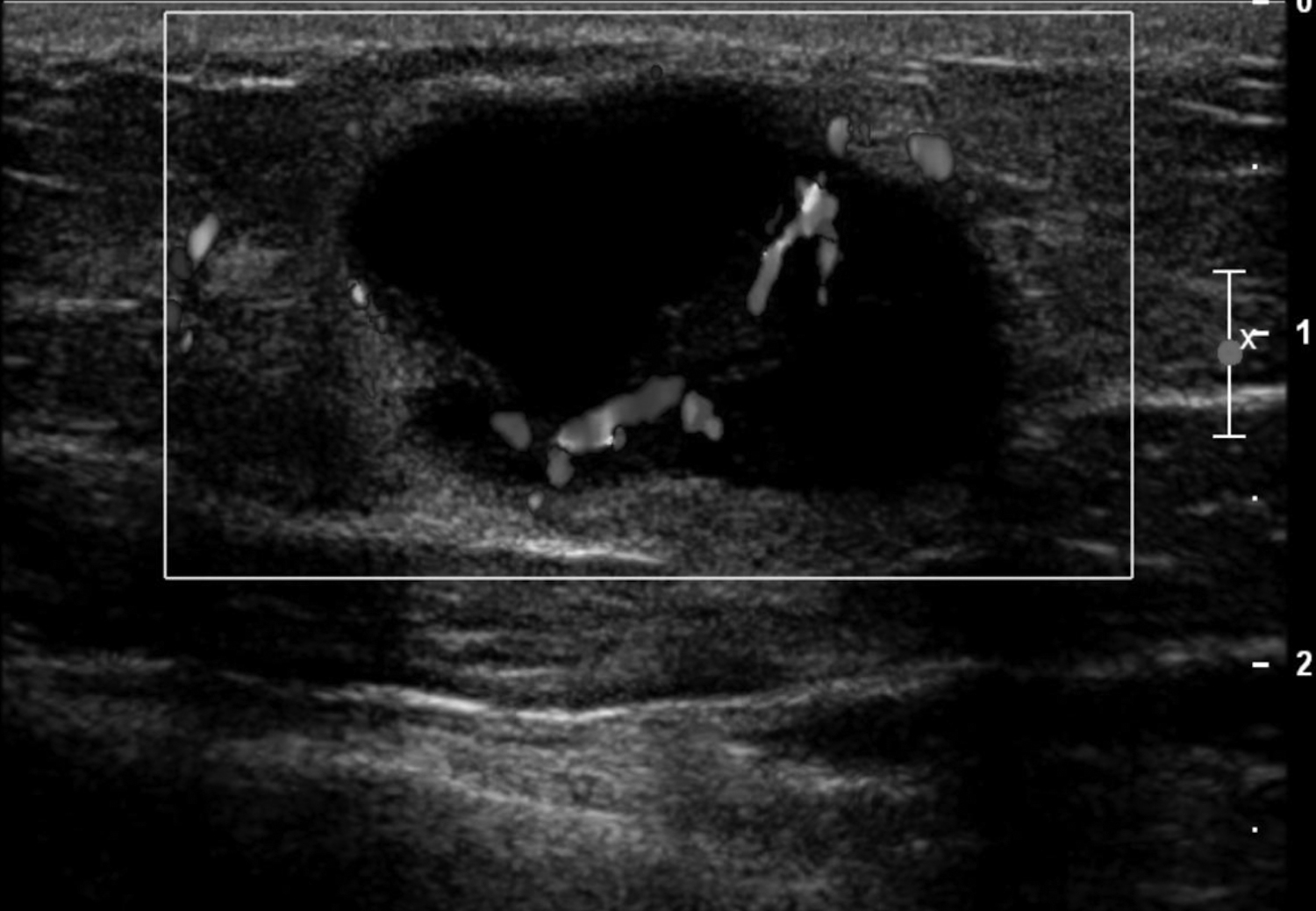
Fluid collections appearing as cystic masses include hematomas and abscesses. The clinical scenario is typically specific so that when paired with imaging, the diagnosis is definitive. Hematomas are associated with trauma, anticoagulation therapy or interventional procedures. The incidence of post core-needle biopsy hematoma is less than 1% when using lidocaine with epinephrine and sufficient compression.5 The sonographic imaging appearance varies with the age of the hematoma.6 Acute hematomas are hypoechoic collections with layering debris (Figure 1A). Over time, the hematoma organizes developing internal echoes depending on the ratio of clotted and fluid blood and can present as a complex mass (Figure 1B).1 Follow-up ultrasound evaluation of hematomas is often recommended to document resolution.2
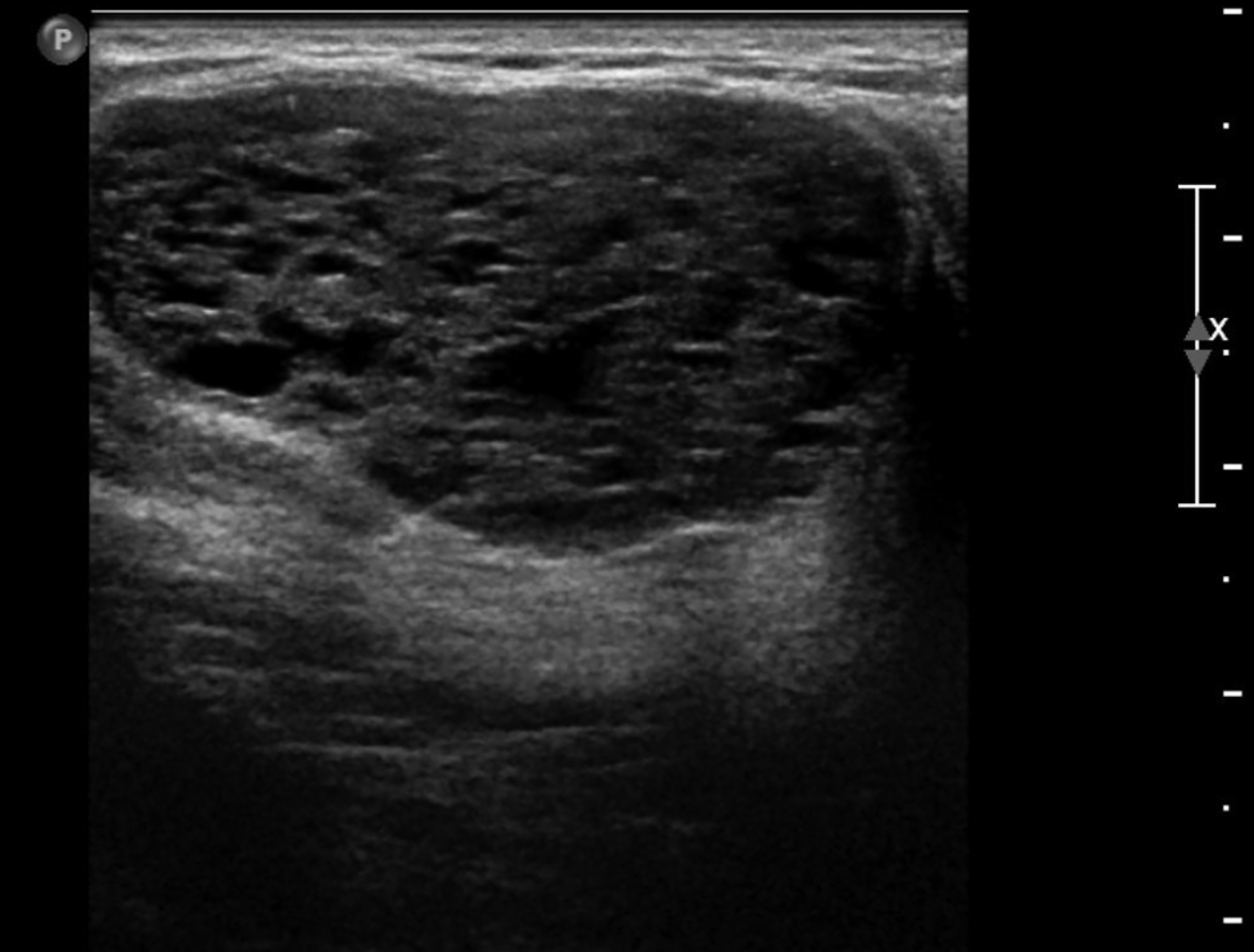
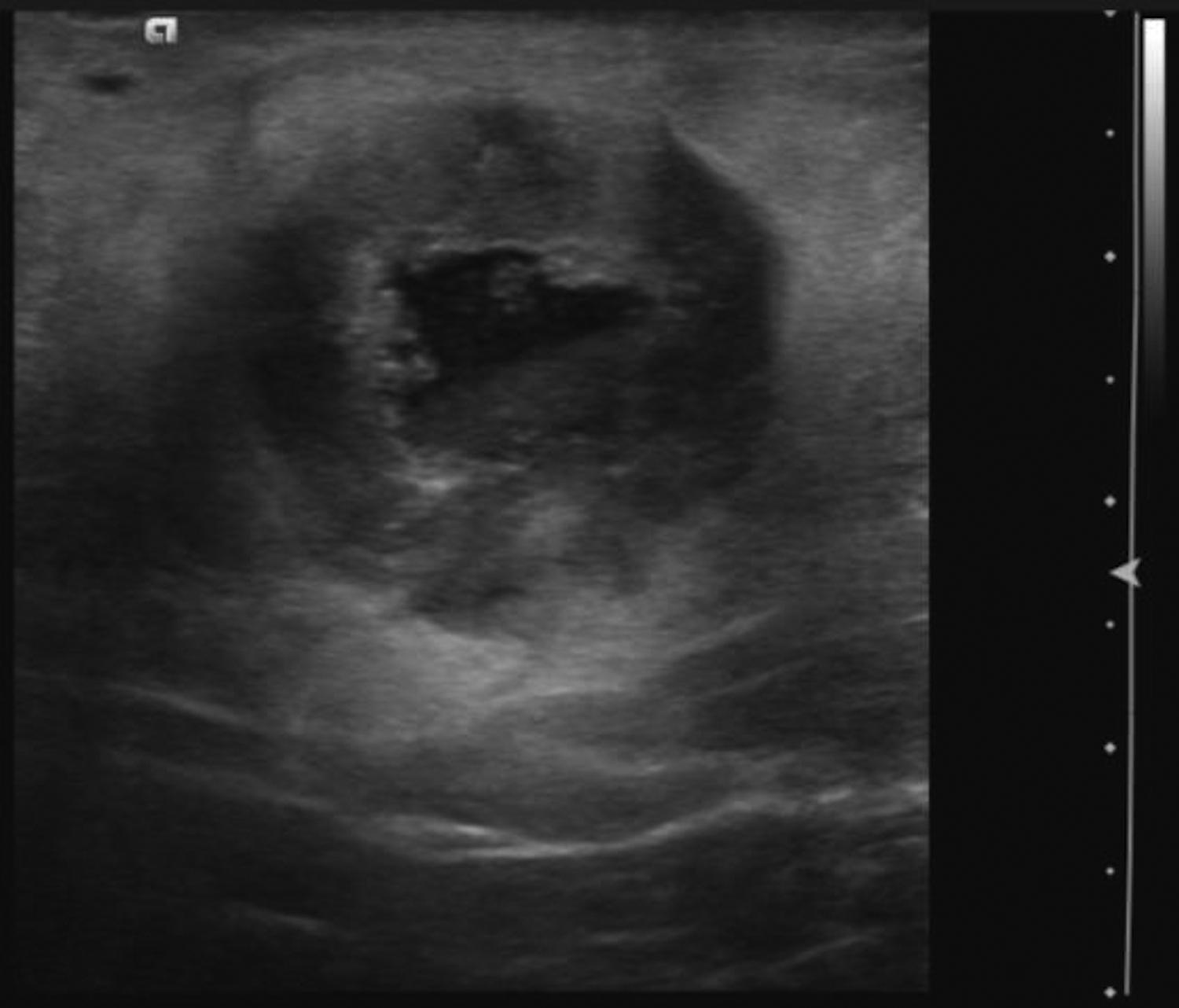
Abscesses are collections of fluid and pus resulting from infection and are more frequent in younger women. They may be related to progressive mastitis or, less commonly, a complication of an interventional procedure. Symptoms include pain, warmth, skin thickening and erythema.1,5Staphylococcus aureus is the most common pathogen.5 Puerperal abscesses occur in 1-24% of lactating women and occur as a complication of mastitis in 5-11% of these women. Non-puerperal abscesses occur in non-lactating women. Puerperal abscesses respond better to treatment.7 Abscesses features on ultrasound are complex multi-loculated fluid collections with internal debris and surrounding hyperemia (Figure 2).5Abscesses are typically managed by antibiotics with or without drainage. Surgical intervention is declining, as percutaneous drainage is less invasive and equally effective, with reported success in 54-100% of patients.7 Follow-up imaging is recommended if the clinical response is prolonged. This is to evaluate for the presence of an underlying malignancy, which may be the cause of the lymphatic or duct obstruction that preceded abscess formation. Rarely, inflammatory breast cancer can result in the development of malignant breast abscesses and a punch biopsy may be necessary for diagnosis.6
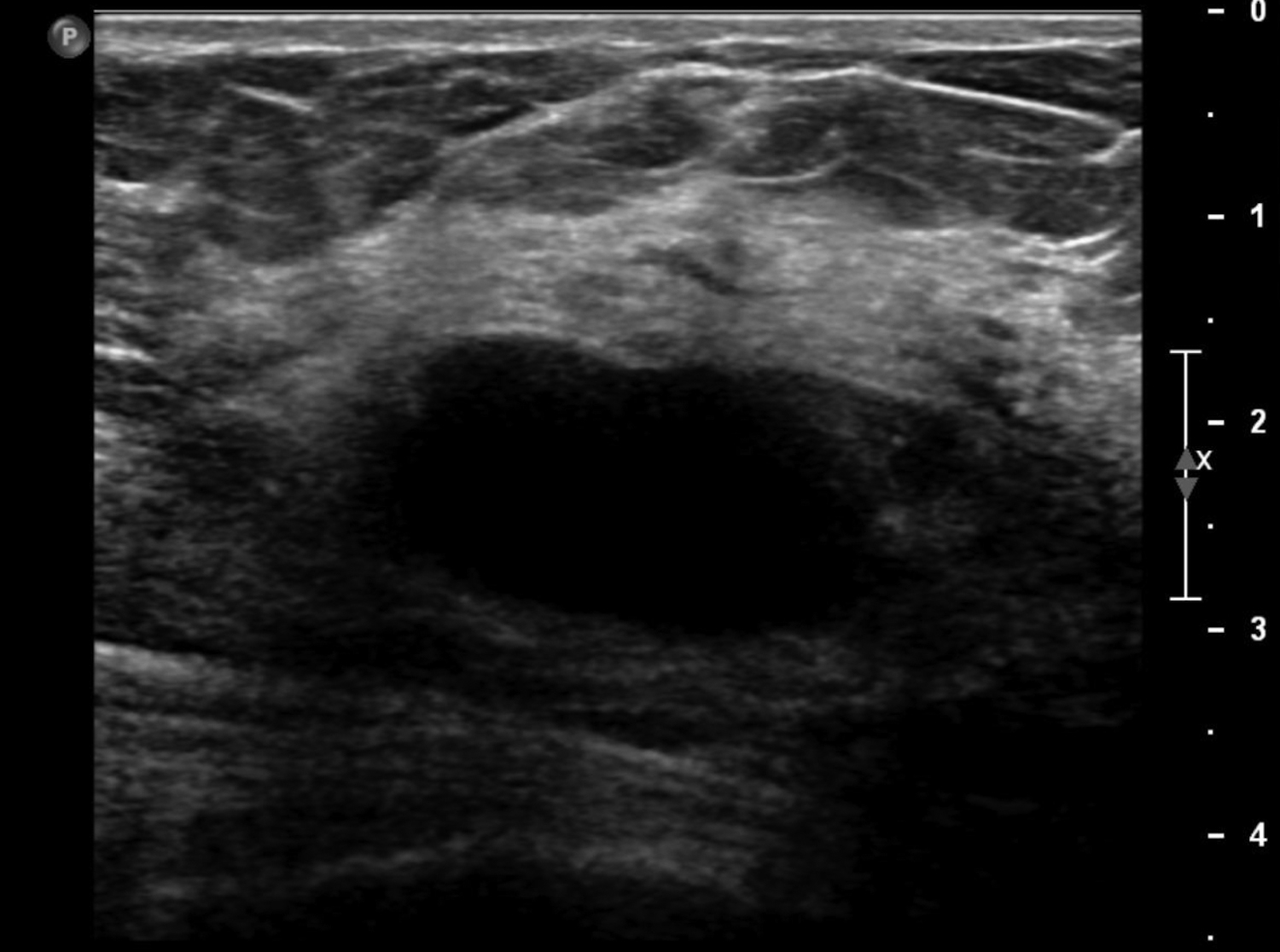
A galactocele is the most common mass seen in women during late pregnancy, lactation, and shortly after cessation of lactation.8 This entity presents as a painless, palpable lump. Ultrasound is the best assessment modality, as mammography is limited in the setting of lactation due to stromal proliferation leading to increased mammographic density. Galactoceles are focal dilations of the ductal system resulting from distal duct obstruction of the TDLU. They are fluid filled, containing differing amounts of proteins, fat, and lactose. 9 Initially, galactoceles appear cystic, and complexity increases over time as fat-fluid levels develop, with the milk eventually curdling, resulting in solid components (Figure 3). A biopsy or aspiration may be required if they present as a complex cystic mass.1
Fat necrosis
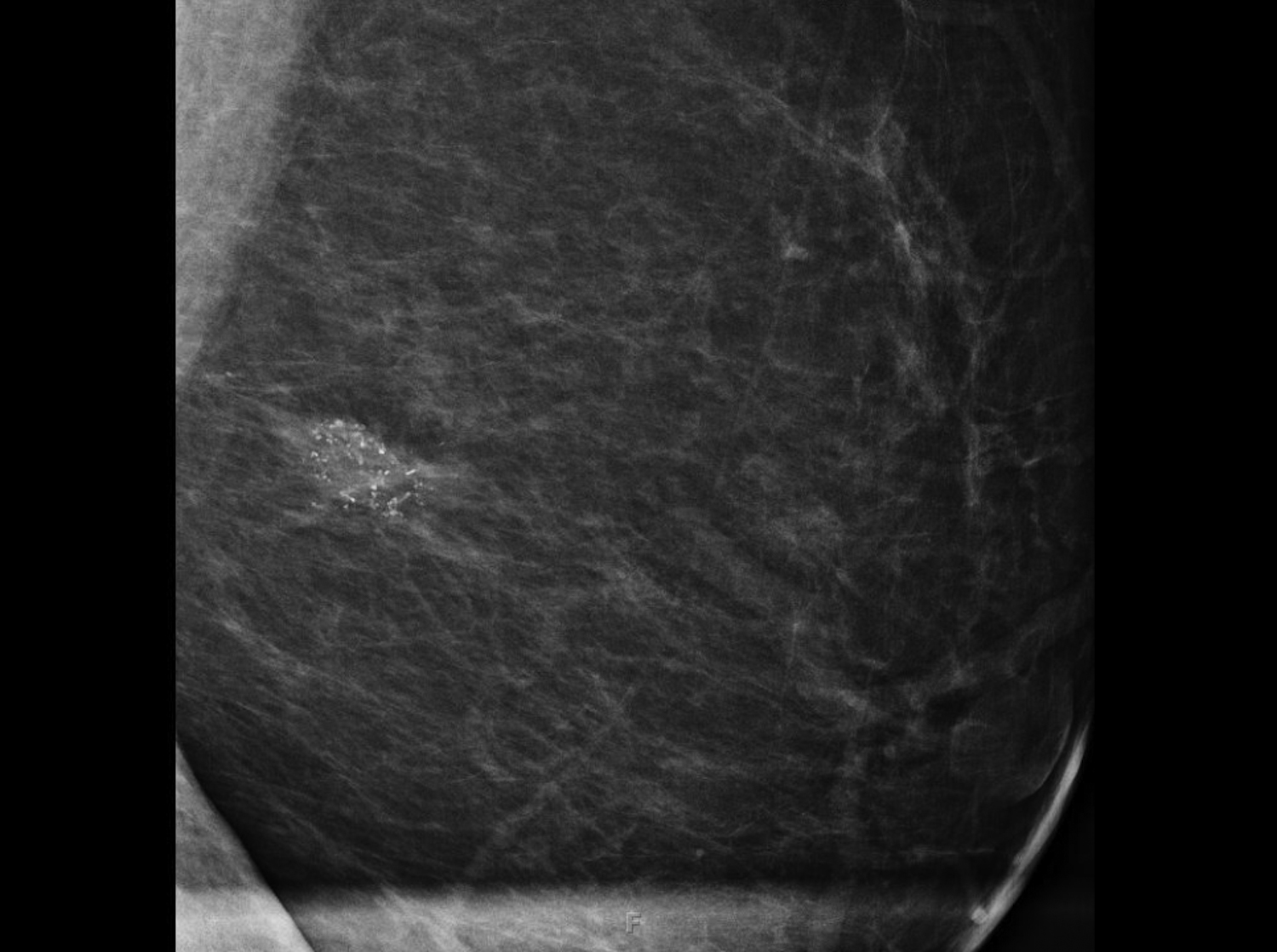
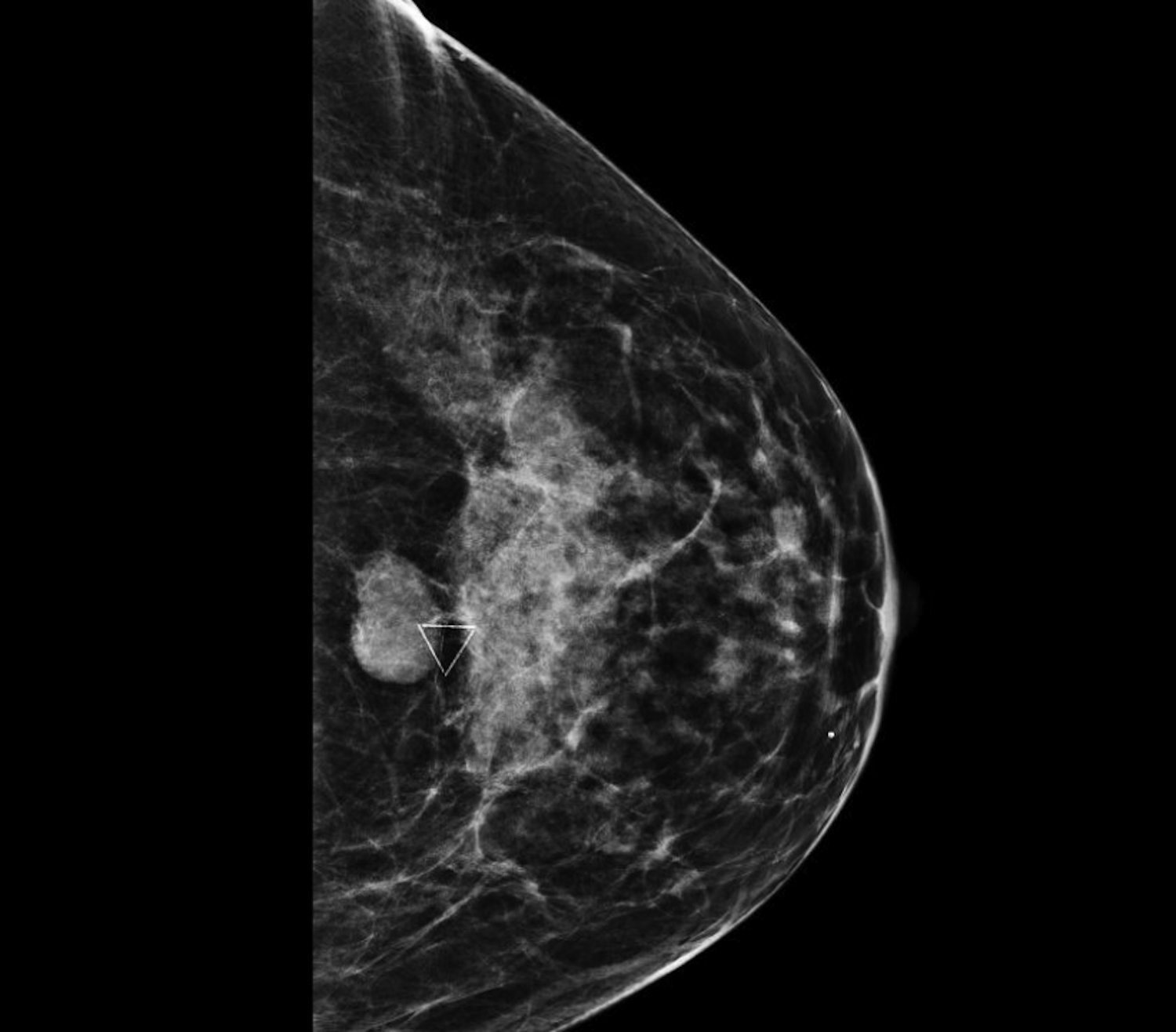
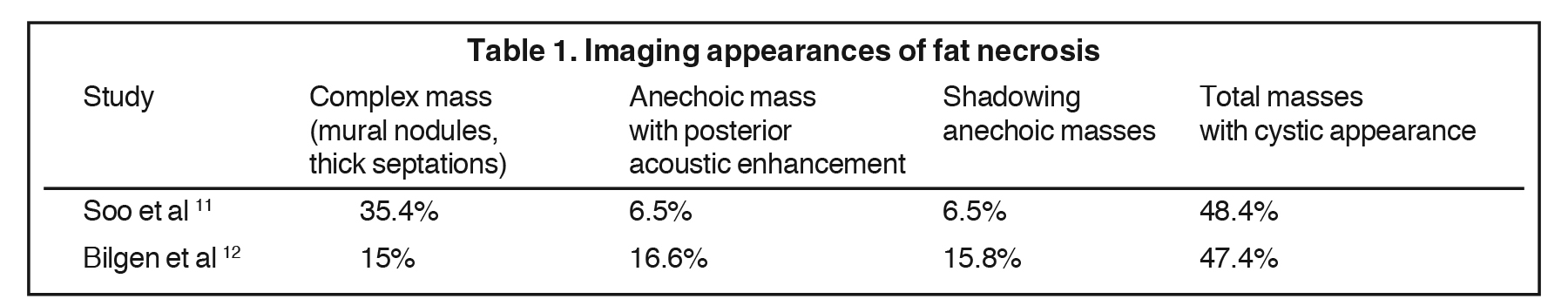
Fat necrosis typically develops after trauma or surgery and frequently occurs as evolution of a hematoma. The degree of trauma may be insignificant enough that many women do not recall the inciting event. Disrupted fat cells and associated hemorrhage result in inflammatory changes that may eventually be replaced by fibrosis. Fat necrosis may result in cystic changes containing oily fluid from necrotic lipid content.1,10 On ultrasound, oil cysts appear as either simple or complicated cysts. As oil cysts evolve, solid components may appear as complex cystic masses on sonographic imaging (Figure 4A).1 Studies have demonstrated fat necrosis to present as cystic masses in as many as 47-48% of cases, as outlined in Table 1.11-12 Echogenic internal bands that change orientation with changes in patient positioning were found to be specific for fat necrosis.11 Mammographically, it may present as a fat-containing mass with calcification (Figure 4B). On MRI, fat necrosis may enhance but with fat signal on all sequences. Correlating ultrasound, mammography and MRI results in the correct diagnosis, and eliminates the need for biopsy.10
Fibroepithelial lesions
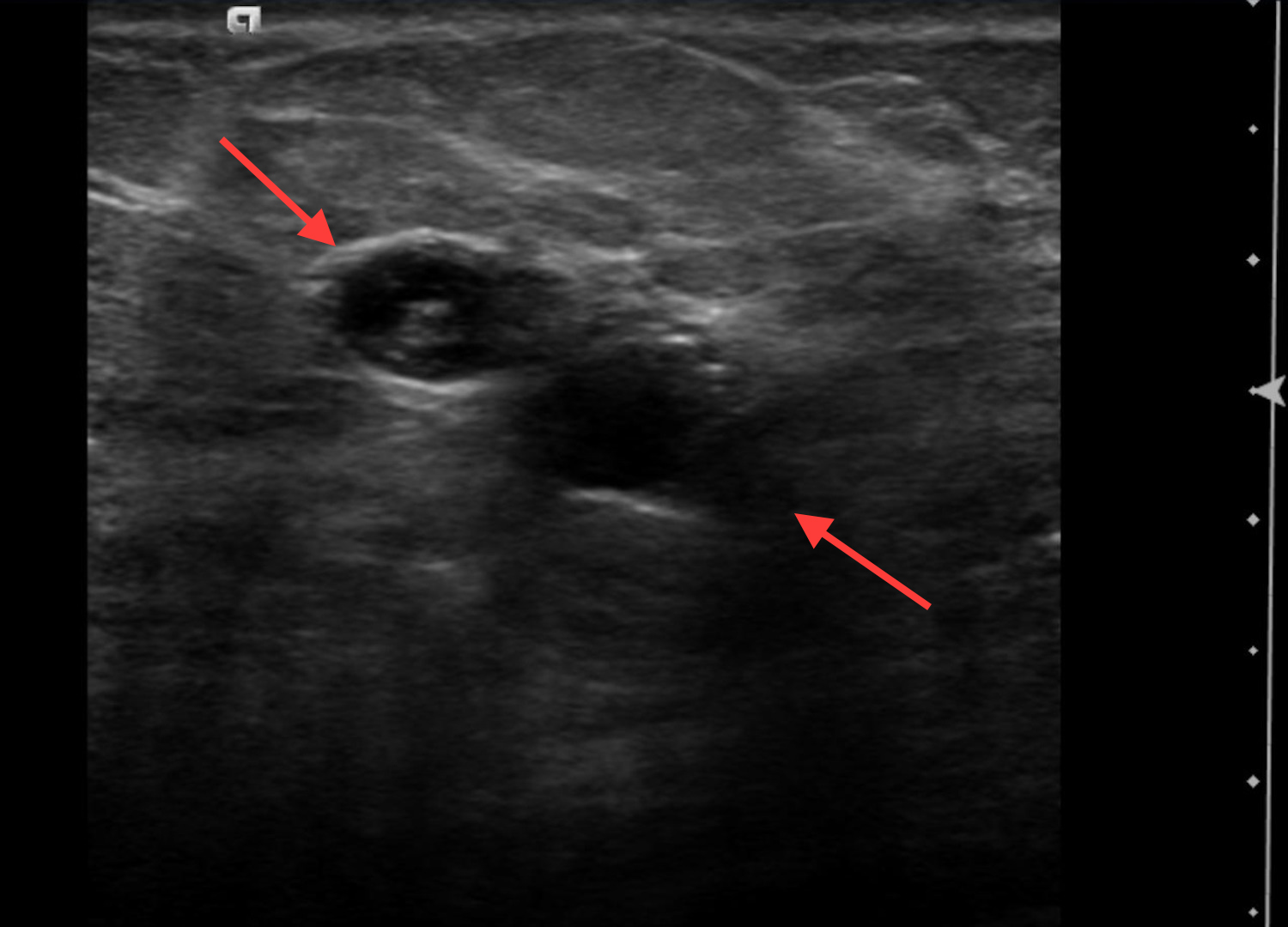
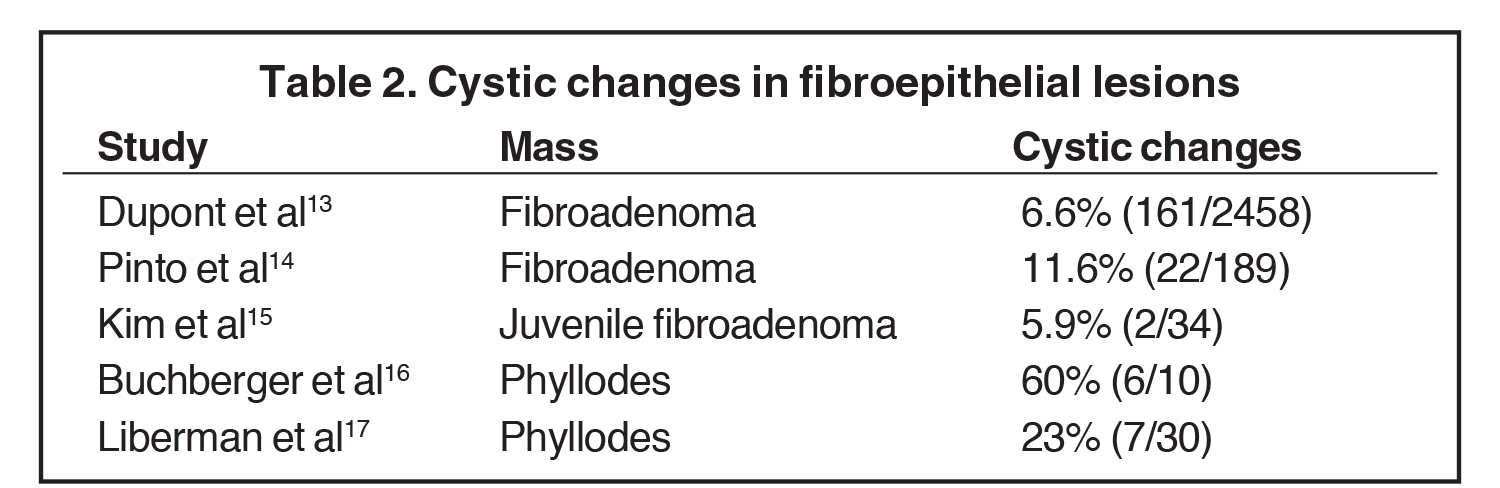
Fibroadenomas are benign neoplasms that develop in the TDLU. On imaging, fibroadenomas may present as solid masses with cystic clefts and less often as a predominantly cystic mass with solid components. The cystic appearance of fibroadenomas ranges from 6.6 -11%.13-14 A cystic appearance may be related to complex histology and Dupont et al found there was a 3.1-increased relative risk of malignancy with complex fibroadenomas compared to simple ones.13 Juvenile fibroadenomas, also known as giant fibroadenomas, are a rare variant, with a prevalence of approximately 2-7.6%. These are typically seen in the teenage years and may also present with cystic changes (Figure 5).15
Phyllodes tumors are uncommon, comprising 0.3%-1.0% of breast neoplasms. These have stromal and epithelial elements, and therefore share similarities with fibroadenomas. Histologic sampling is required to differentiate benign and malignant tumors, and surgical resection may be needed as well. The local recurrence rate ranges from 16-28% secondary to incomplete excision; therefore, wide margins are needed to prevent recurrence. Approximately 29% of malignant tumors develop metastases.16 There is a wide range in the percentage of phyllodes presenting with cystic changes. Liberman et al found that cystic changes were present in more malignant tumors than benign, but the difference was not statistically significant.17 Buchberger et al concluded that cystic changes were not pathognomonic and found biopsy was needed.16 Studies describing incidence of cystic changes in these fibroepithelial lesions are outlined in Table 2.13-17
Papillary and high-risk cystic breast lesions
Several pathologies are classified as high-risk lesions. These are managed with surgical excision, as histopathologic assessment of the entire lesion may show associated malignancy. These pathologies include: atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS) and atypical papillomas, all of which may present as cystic lesions in the breast.2
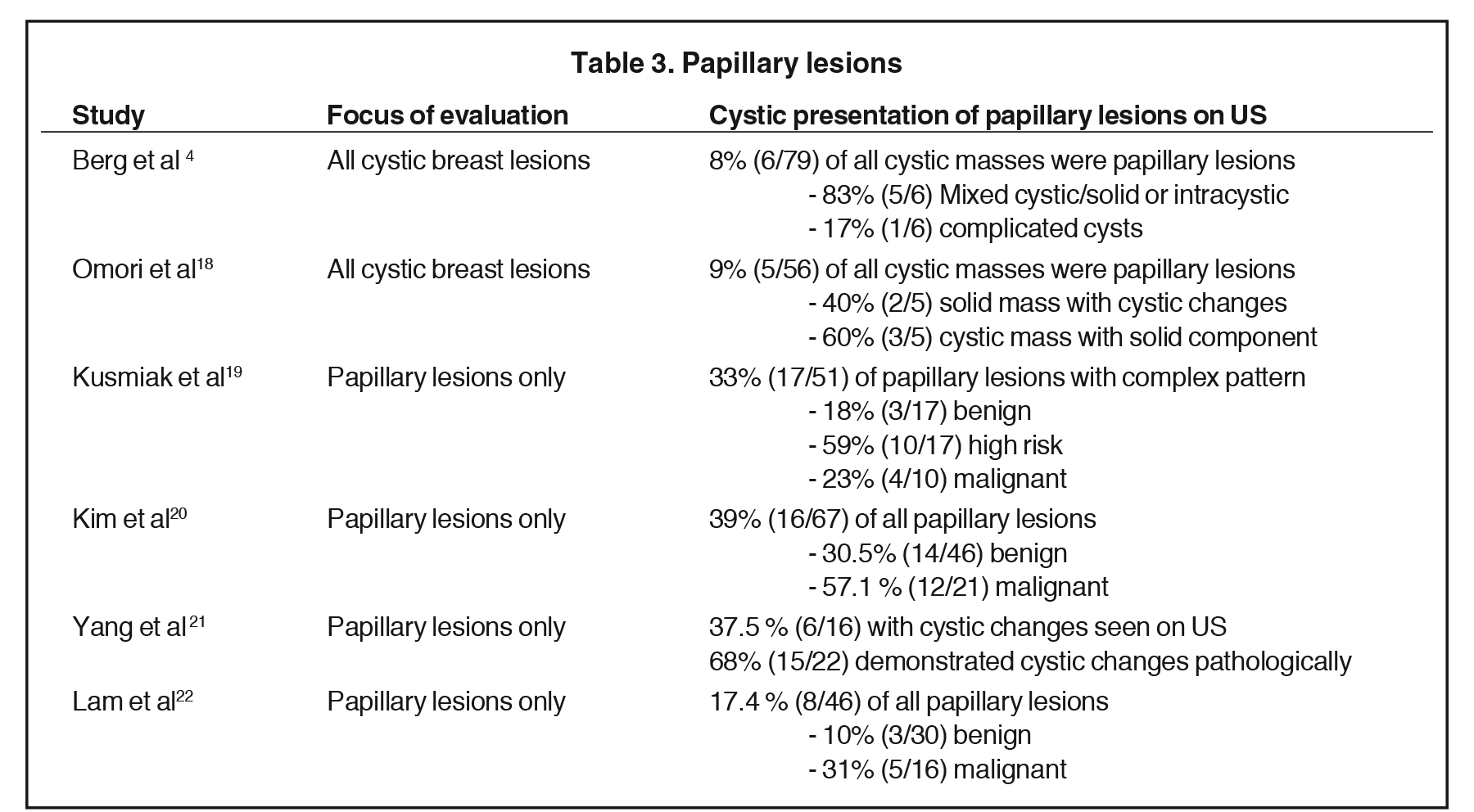
Of these pathologies, papillary lesions are the most likely to present as cystic masses. Papillomas often come to attention due to clear or bloody nipple discharge and can be benign, associated with high-risk lesions and/or malignancy. Papillomas can obstruct ducts and secrete fluid, forming cystic spaces. Papillomas may appear on ultrasound as intraductal masses, complex solid and cystic masses or solid masses without associated duct ectasia (Figure 6).1Studies have demonstrated that 8-9% of all cystic breast lesions are found to be papillomas.4,18 Additional studies focusing on papillary lesions demonstrate a wide range of cystic changes, as outlined in Table 3.4,18- 22 Yang et al found that 37.5% (6/16) of the papillomas seen on ultrasound presented with cystic components; however, pathologically 68% (15/22) demonstrated cystic changes. These cystic papillary lesions commonly demonstrated mural thickening or internal echogenic tissue that occasionally demonstrated vascularity.21 Thus, papillomas are included in the differential of cystic breast lesions.
Malignant cystic breast lesions
Primary breast malignancies
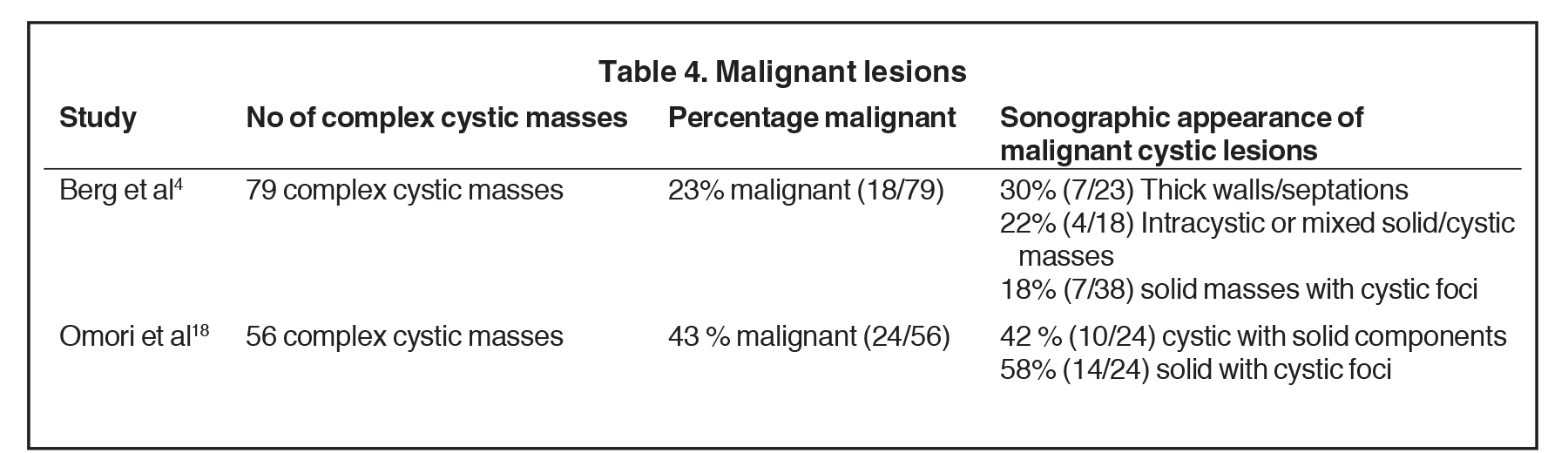
Infiltrating ductal carcinoma (IDC) and ductal carcinoma in situ (DCIS) are the most common malignancies to present as complex cystic and solid masses.2 Of invasive ductal malignancies, grade 3 cancers may present as cystic masses with posterior acoustic enhancement due to their increased cellularity mimicking a benign lesion (Figure 7).23In papillary DCIS, malignant cells grow with papillary projections into the duct (Figure 8). This variant is associated with a higher rate of microinvasion and multicentric disease in comparison to other DCIS.24 Infiltrating lobular carcinoma (ILC) accounts for 7-10% of breast malignancies. Invasive malignancies typically present as irregular masses and less commonly, can present as complex cystic masses. Suspicious masses may present with thick walls and thick septations (Figure 9) or as solid masses with cystic foci.25 Studies have demonstrated a wide range in the incidence of primary breast malignancy presenting as cystic masses, as outlined in Table 4.4,18 Of 18 that were found malignant by Berg et al, the pathologies were the following: 50% IDC, 22% IDC/DCIS, 17% DCIS, 6% ILC, and 6% a combination of IDC, DCIS and ILC (Figure 10).4 Therefore, the percentage of malignancies presenting as complex cystic and solid masses varies, but it is high enough that ultrasound-guided biopsy is warranted.
Papillary carcinomas represent only 1-2% of all breast malignancies. They occur mostly in postmenopausal women, often present with nipple discharge, and have a good prognosis, as they are well differentiated. Papillary carcinomas are seen on ultrasound as solid masses, complex cystic and solid lesions or intracystic masses (Figure 11). Intracystic papillary carcinomas are seen in fluid-filled ectatic ducts as a vascular solid component. As these are vascular, hemorrhage may occur, resulting in fluid-debris levels on ultrasound imaging.24 In the study by Berg et al, 75% of the malignant intracystic masses were papillary in origin.4 Additional studies have shown papillary malignancies present as complex cystic masses in 24-57% of cases as outlined in Table 3.4, 18-22
Mucinous carcinomas, also called colloid carcinomas, are most common in older women, associated with a good prognosis these lesions comprise 1-7% of invasive breast cancers.26 Mucinous carcinomas often appear as hypoechoic to anechoic, round, circumscribed masses on ultrasound and as dense round, circumscribed masses on mammography.27-28 In a study by Lam et al, 37.5% presented as a complex mass with solid and cystic components and were more common in mixed variants and grade 1 tumors.29 Other studies however were unable to correlate sonographic features with histologic grade (Figure 12).27
Metastases
Metastatic disease to the breast often indicates late-stage disease and has a poor prognosis. Clinical history is critical, as a primary malignancy or other metastatic lesions narrow the differential diagnosis. The most common origins of metastatic disease from extra-mammary malignancies include lymphoma/leukemia and melanoma. Metastatic disease to the breast is uncommon with incidence ranging from 1.7-6.6% in autopsies series, with variable rates dependent on whether lymphoma/leukemia were included in the studies. Additional studies have demonstrated the clinical incidence to average around approximately 2%. 30
Metastatic lesions are most commonly located in the upper outer quadrant30 typically in superficial breast tissue with rich blood supply.31 Metastases can spread to the breast via hematologic or lymphatic pathways. The hematogenous metastases are more likely to form breast masses. Metastases have a variety of presentations, most commonly seen on ultrasound as solid hypoechoic masses with circumscribed margins. Imaging is often unable to distinguish metastases from other processes.32 Breast metastases can present as cystic masses on ultrasound with reports of intratumoral cystic lesions.31 In a study by Lee et al, 21% of patients had breast metastases from an extramammary malignancy presenting as a cystic mass with complex echo pattern. 80% of these lesions were lymphoma metastases and 20% from malignant fibrous histocytoma .32 Other metastatic lesions to the breast include lung, ovarian and gastric carcinomas (Figure 13).33 In another institution, metastatic disease with cystic foci was seen in patients with synovial sarcoma, hepatocellular carcinoma and insular carcinoma of the thyroid gland.31 Cystic metastases are uncommon, but regardless of the imaging presentation, biopsy is required for final diagnosis.
Conclusion
Cystic breast disease encompasses a large differential diagnosis. The differential varies between masses that are predominantly solid with cystic foci and masses that are predominantly cystic with solid components. As a result, the lack of specificity in imaging findings often requires biopsy with histopathologic analysis for final diagnosis.
References
- Hines N, Slanetz PJ, Eisenberg RL. Cystic masses of the breast. AJR Am J Roentgenol. 2010; 194(2):122-133.
- Doshi DJ, March DE, Crisi GM, et al. Complex cystic breast masses: diagnostic approach and imaging-pathologic correlation. Radiographics. 2007; 27 (1): 53-64.
- D’Orsi CJ, Sickles, EA, Mendelson EB, et al. ACR BI-RADS Atlas Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology, 2013.
- Berg WA, Campassi CL, Ioffe OB. Cystic lesions of the breast: sonographic-pathologic correlation. Radiology. 2003; 227(1):183-191.
- Mahoney MC, Ingram AD. Breast emergencies: types, imaging features, and management. AJR Am J Roentgenol. 2014; 202(4): 390-399.
- Yiming G, Slanetz P, Eisenberg R. Echogenic breast masses at US: to biopsy or not to biopsy. Radiographics. 2013; 33(2):419-434.
- Trop I, Dugas A, Julie D, et al. Breast abscesses: evidence-based algorithms for diagnosis, management, and follow-up. Radiographics. 2011; 31(6):1683-1699.
- Son EJ, Oh KK, Kim EK. Pregnancy-associated breast disease: radiologic features and diagnostic dilemmas. Yonsei Med J. 2006; 47(1):34-42.
- Sabate JM, Clotet M, Torrubia S, et al. Radiologic Evaluation of Breast Disorders Related to Pregnancy and Lactation. Radiographics. 2007; 27(1) :101-124.
- Taboada JL, Stephens TW, Krishnamurthy S, et al. The Many Faces of Fat Necrosis. AJR Am J Roentgenol. 2008; 192(3): 815-825.
- Soo MS, Kornguth PJ, Hertzberg BS. Fat necrosis in the breast: sonographic features. Radiology. 1998; 206(1): 261-269.
- Bilgen IG, Ustun EE, Memis A. Fat necrosis of the breast: clinical, mammographic and sonographic features. Eur J Radiol. 2001; 39(2): 92-99.
- Dupont WD, Page DL, Parl FF, et al. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994; 331(1): 10-15.
- Pinto J, Aguiar AT, Duarte H, et al. Simple and complex fibroadenomas: are there any distinguishing sonographic features? J Ultrasound Med. 2014; 33(3): 415-419.
- Kim SJ, Park YM, Jung SJ, et al. Sonographic appearances of juvenile fibroadenoma of the breast. J Ultrasound Med. 2014; 33(11):1879-1884.
- Buchberger W, Strasser K, Heim K, et al. Phyllodes tumor: Findings on mammography, sonography and aspiration cytology in 10 cases. AJR Am J Roentgenol. 1991; 157(4): 715-719.
- Liberman L, Bonaccio E, Hamele-Bena D, et al. Benign and malignant phyllodes tumors: mammographic and sonographic findings. Radiology. 1996; 198(1): 121-124.
- Omori LM, Hisa N, Ohkuma K, et al. Breast masses with mixed cystic-solid sonographic appearance. J Clin Ultrasound, 2005; 21(8):489-495.
- Kuzmiak CM, Lewis MQ, Zeng D, et al. Role of sonography in the differentiation of benign, high-risk, and malignant papillary lesions of the breast. J Ultrasound Med. 2014; 33(9):1545-1552.
- Kim TH, Kang DK. Kim SY, et al. Sonographic differentiation of benign and malignant papillary lesions of the breast. J Ultrasound Med, 2008; 27(1):75-82.
- Yang WT, Suen M, Metreweli C. Sonographic features of benign papillary neoplasms of the breast: review of 22 patients. J Ultrasound Med. 1997; 16(3):161-168.
- Lam WW, Chu WC, Tang AP, et al. Role of radiologic features in the management of papillary lesions of the breast. AJR Am J Roentgenol. 2006; 186(5):1322-1327.
- Lamb PM, Perry NM, Vinnicombe SJ, et al. Correlation between ultrasound characteristics, mammographic findings and histologic grade in patients with invasive ductal carcinoma of the breast. Clin Radiol. 2000;55(1):40-44.
- Jagmohan P, Pool FJ, Putti TC, et al. Papillary lesions of the breast: imaging findings and diagnostic challenges. Diagn Interv Radiol. 2013;19(6):471-478.
- Butler RS, Venta LA, Wiley EL, et evaluation of infiltrating lobular carcinoma. AJR Am J Roentgenol. 1999; 172(2):325-330.
- Toikkanen S, Kujari H. Pure and mixed mucinous carcinomas of the breast: a clinicopathologic analysis of 61 cases with long-term follow-up. Hum Pathol. 1989; 20(8): 758-764.
- Liu H, Tan H, Cheng Y, et al. Imaging findings in mucinous breast carcinoma and correlate factors. Eur J Radiol. 2011; 80(3): 706-712.
- Conant EF, Dillon RL, Palazzo J, et al. Imaging findings in mucin-containing carcinomas of the breast: correlation with pathologic features. AJR Am J Roentgenol. 1994; 163(4):821-824.
- Lam WW, Chu WC, Tse GM, et al. Sonographic appearance of mucinous carcinoma of the breast. AJR Am J Roentgenol. 2004; 182(4):1069-1074.
- Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977; 129(4):673-676.
- Mun SH, Ko EY, Han BK, et al. Breast metastases from extramammary malignancies: yypical and atypical ultrasound features. Korean J Radiol. 2014; 15(1): 20-28.
- Lee JH, Kim SH, Kang BJ, et al. Metastases to the breast from extramammary malignancies- sonographic features. J Clin Ultrasound. 2011; 39(5): 248-255.
- Lee SH, Park JM, Kook SH, et al. Metastatic tumors to the breast: mammographic and ultrasonographic findings. J Ultrasound Med. 2000; 19(4):257-262.
Citation
PG V, A S, K F, S H. Cysts with masses and masses with cysts: An imaging review of cystic breast masses. Appl Radiol. 2017;(10):8-18.
October 6, 2017