Contrast-Enhanced US for Characterization and Biopsy of Indeterminate Hepatic Lesions and Metastases: A Review with Case Examples
During the past decade, there have been significant advances in systemic therapy for oncologic disease. Most notably, immunotherapeutics have prolonged survival in patients with previously untreatable, advanced metastatic disease.1 Concurrently, innovations in medical imaging have made radiologic exams increasingly sensitive in detecting metastases,2, 3 and well-elucidated imaging criteria, such as RECIST, have been validated for assessing treatment response.4 These factors have led to significant increases in the volume and frequency of oncologic imaging exams.5, 6
Imaging Techniques
Contrast-enhanced CT (CECT) is the mainstay of oncologic imaging, where it is used for disease staging and surveillance.7 While the appearance of metastases varies based on the primary malignancy and site of metastasis, many visceral metastases have classic features that may obviate the need for biopsy.8, 9 The liver, one of the most common sites for metastatic disease, is well visualized on CECT scans, allowing for effective metastasis detection.10 Nonetheless, the variable appearance of metastatic lesions and the possibility of benign mimics can make definitive characterization of hepatic lesions impossible.11, 12
Most hepatic metastases appear hypoenhancing on CECT, with colorectal, lung, and breast cancer metastases serving as classic examples.9, 10 As lesions grow, central necrosis can give lesions a rim-enhancing appearance with a nonenhancing liquid center.9 Conversely, hemangioma, the most commonly encountered benign hepatic neoplasm, exhibits progressive peripheral nodular enhancement, with fill-in on delayed phases.12
A minority of hepatic metastases appear hyperenhancing and are most conspicuous on arterial phase exams. These include melanoma, renal cell carcinoma, and neuroendocrine tumors.9, 12 While these lesions tend to exhibit washout in the latter phases, distinguishing them from benign entities such as focal nodular hyperplasia or adenoma can be difficult without prior imaging to confirm lesion stability.12 Furthermore, this imaging appearance may be indistinguishable from well-differentiated hepatocellular carcinoma, though a benign neoplasm would be statistically favored in the absence of cirrhosis.12
Increasingly, hepatic metastases are being detected with highly sensitive, advanced imaging techniques like MRI with diffusion-weighted imaging, FDG-PET, or targeted radionuclide SPECT.13, 14 MRI is particularly useful in detecting and surveilling liver metastasis. On T2 sequences, these metastases are typically mildly T2 hyperintense, with possibly moderate central T2 signal intensity in the presence of concurrent cystic or necrotic changes. This contrasts with the marked T2 hyperintensity of many benign lesions. T1 hyperintensity varies and reflects the histologic characteristics of the underlying metastatic lesion. Gradient echo sequences allow detection of iron and fat deposition within focal lesions, which can further aid lesion characterization.
Hepatobiliary contrast agents (HBAs) have increased sensitivity in detecting liver metastasis. These agents can exclude primary hepatic lesions since metastatic lesions will appear hypointense on delayed HBA sequences owing to the absence of functional hepatocytes. One meta-analysis found that Gd-EOB-DTPA demonstrated a higher per-lesion sensitivity than CECT, a median of 94.9% versus 74.2%, respectively.15 On diffusion-weighted imaging, metastases commonly exhibit low apparent diffusion coefficient values, reflecting high cellularity, with a reported sensitivity of 87% for liver metastases.16
Ultrasound, while used less often in cancer surveillance due to reduced sensitivity,17 plays a significant role in percutaneous image-guided liver biopsy. It imparts no radiation dose to the patient and provider, allowing continuous real-time imaging. This capability aids precise lesion localization and identification of sensitive surrounding structures, ensuring a safer biopsy approach.18
The use of contrast-enhanced US (CEUS) for the assessment and sampling of hepatic metastases has been demonstrated in multiple case series.19 - 21 This modality employs microbubble-based agents, which are composed of a fluorinated gas core enveloped by a phospholipid shell. The gas core is exhaled through the lungs, while the phospholipid component is metabolized by the liver, bypassing renal excretion. Lesions typically exhibit enhancement similar to that seen on cross-sectional imaging, but their enhancement characteristics may be better elucidated given the superior temporal resolution afforded by continuous image acquisition.22 These data can be used to generate kinetics curves, which aid delineation of benign and malignant lesions and, in the case of heterogeneous tumors, identify areas of rapid enhancement and washout, which are predictive of aggressive tumor histology.23 Additionally, like other contrast-enhanced techniques, CEUS can reliably distinguish enhancing tumor from nonenhancing regions of necrosis and has been found to increase single-puncture success rate and diagnostic accuracy.19
At our institution, US contrast is administered for biopsy in select cases where lesions are sonographically occult or exhibit significant heterogeneity on grayscale US.
The following 4 cases illustrate the utility of CEUS for hepatic biopsies.
Case No. 1: Colon Cancer
An elderly patient with a history of metastatic colon cancer was referred to radiology for biopsy of growing hypoattenuating hepatic lesions seen on surveillance CT exam ( Figure 1 ). The patient had previously been treated with a chemotherapy regimen of FOLFOX and bevacizumab, which was discontinued owing to enlargement of the presumed metastatic lesions. Biopsy was requested to assess eligibility for an immunotherapy research trial.
( A ) Contrast-enhanced CT demonstrates multiple hypoattenuating lesions within the right hepatic lobe (arrows). ( B ) Contrast-enhanced US demonstrates a lobulated subcapsular lesion corresponding to the larger, hypoattenuating lesion seen on CT, with thick peripheral enhancement (arrows) and hypoechoic center (white arrowheads), presumed to represent necrosis. ( C ) Subtle corresponding contour (black arrowhead) is seen on grayscale US.
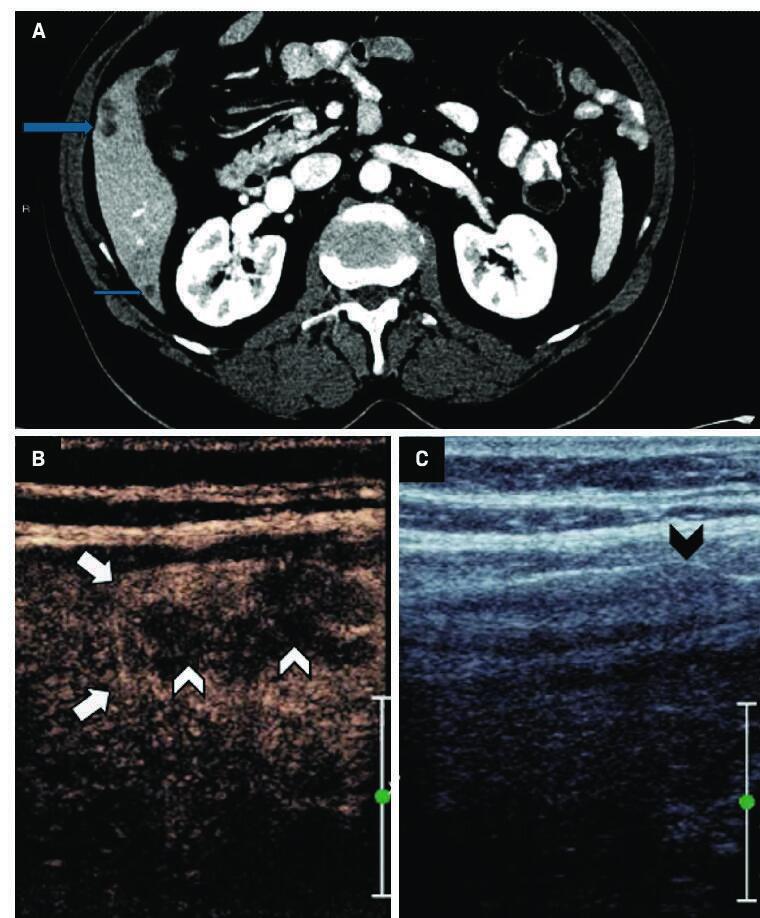
Initial grayscale US demonstrated a subtle contour bulge, which was much less conspicuous than that corresponding to the dominant hypoattenuating lesion seen on CT. Administration of 2 mL of Lumason (sulfur hexafluoride lipid-type A microspheres) revealed a lobulated lesion with persistent, thick peripheral enhancement and a central nonenhancing component, presumed to represent necrosis. Based on the CEUS, the lesion’s periphery was targeted for percutaneous biopsy. Initial 25G fine-needle aspiration (FNA) with on-site cytopathology was suspicious for metastatic adenocarcinoma. Multiple 18G core biopsies later confirmed the diagnosis and were sent for immunohistochemical analysis for potential clinical trial enrollment. The patient tolerated the procedure well and was discharged home following a brief recovery.
Case No. 2: Neuroendocrine Tumor
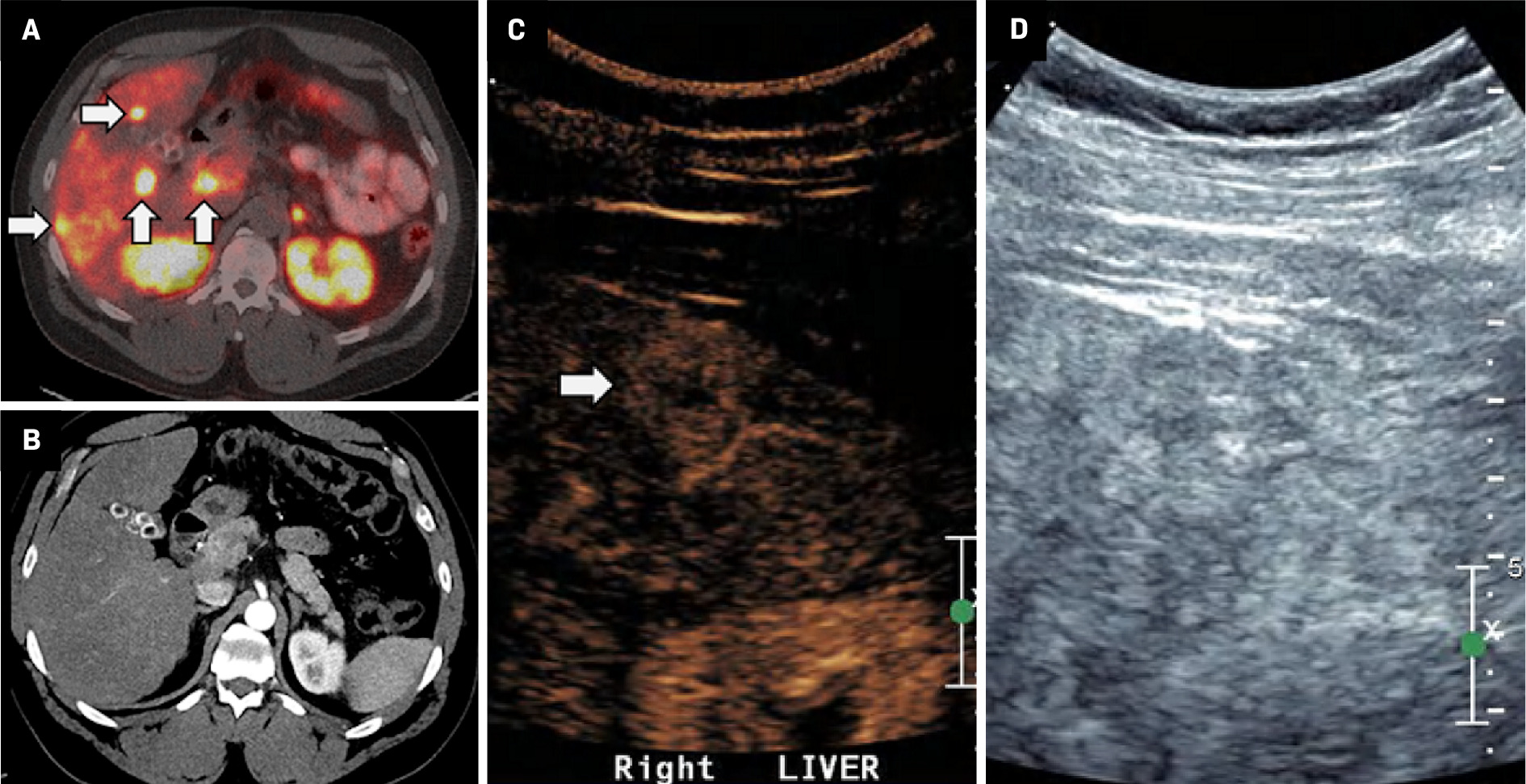
An adult with metastatic neuroendocrine tumor of the small bowel was referred for biopsy of presumed hepatic metastases. The patient had previously received treatment with somatostatin analogs (octreotide LAR, Lutathera) and sunitinib and had relatively stable disease until their most recent DOTATE-PET/CT ( Figure 2 ), which showed multiple radiotracer-avid hepatic lesions growing in size and number. The patient was presumed to have progressive metastatic disease in the setting of continued therapy, raising concern for tumor mutation/dedifferentiation. Multiphase CECT demonstrated no correlation to the radiotracer-avid hepatic lesions. Subsequent CEUS (2 mL Lumason, 2 boluses) revealed a subtle, hyperenhancing, subcapsular right hepatic lobe lesion corresponding to a radiotracer-avid focus on DOTATE-PET/CT. 25G FNA revealed a metastatic neuroendocrine tumor, and multiple 18G core biopsies were obtained for immunohistochemical staining, which demonstrated an increase in Ki67 proliferation index. Based on these results, therapy was initiated with everolimus, a kinase inhibitor.
( A ) DOTATE-PET demonstrates multiple radio-avid hepatic metastases (white arrows), occult on ( B ) corresponding contrast-enhanced CT.32id focus (black arrow). ( D ) A mildly hypoechoic correlate, noted on grayscale US (arrowhead), is difficult to identify prospectively given the heterogeneous appearance of the liver.
Case No. 3: Pseudolesion
An adult with a history of choroidal ocular melanoma presented with an indeterminate, mildly T2 hyperintense, right hepatic lobe lesion seen on MRI ( Figure 3 ). The patient had previously been treated with light-activated AU-011 conjugated nanoparticles and had no history of metastatic disease. Given the patient’s high-risk disease, the clinical significance of a metastatic lesion, and that the lesion was occult on grayscale US, a CEUS biopsy was performed.
( A ) MRI demonstrates a mildly T2 hyperintense right hepatic lobe lesion (white arrow). ( B ) Contrast-enhanced US at 30 seconds demonstrates a corresponding rim-enhancing lesion (black arrow). ( C ) Lesion is isoechoic at 60 seconds and ( D ) occult on greyscale US.
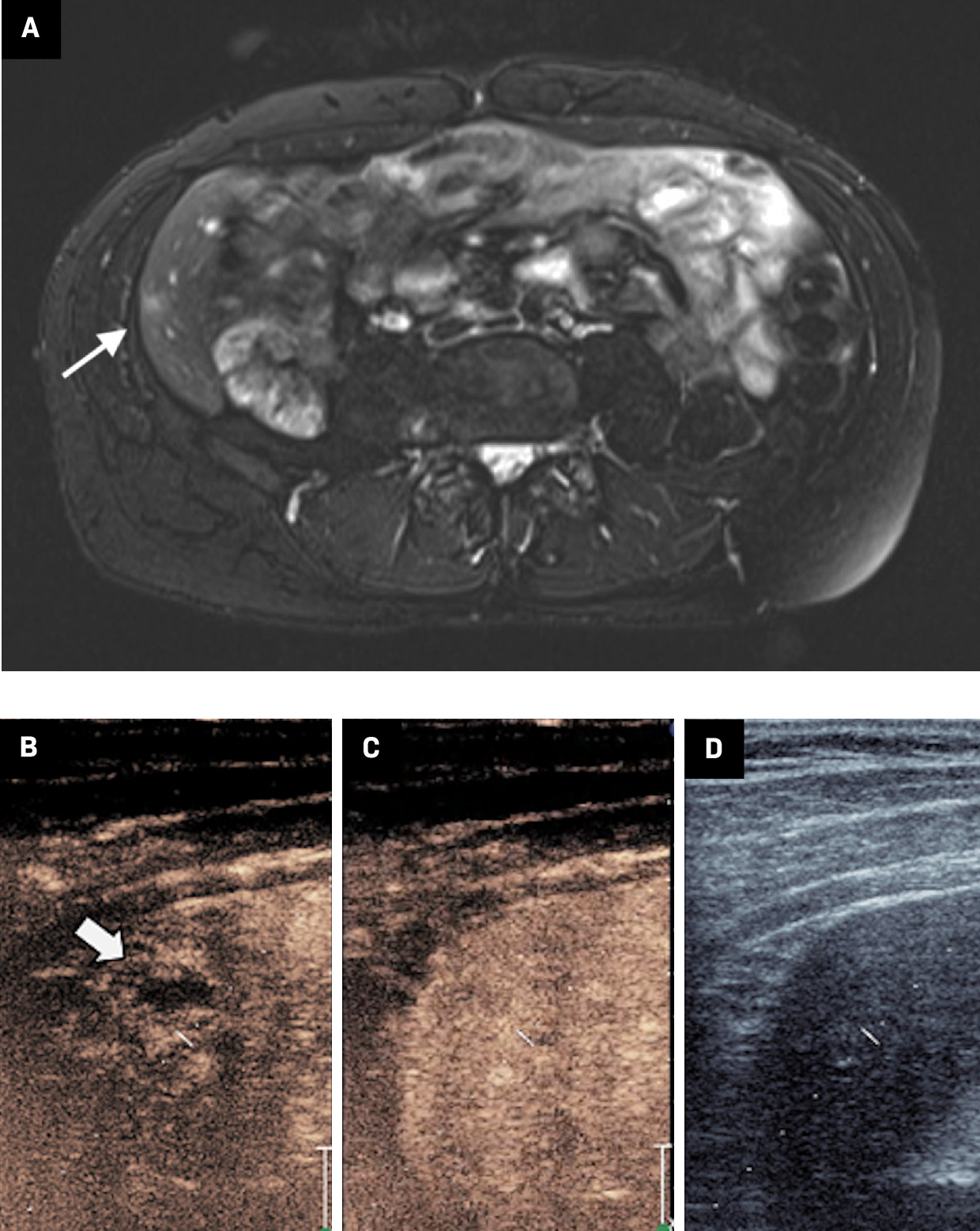
The lesion was identified using anatomic landmarks, and approximately 30 seconds post-administration of 2 mL of US contrast (Lumason), a rim-enhancing lesion was seen. The lesion became isoechoic on subsequent images and was indistinguishable from the liver parenchyma at 60 seconds. While apparent progressive enhancement is atypical for melanoma metastases, the lesion remained indeterminate, and the early-enhancing periphery was targeted for biopsy. Multiple 18G core biopsy samples were obtained, revealing benign hepatocytes with a background of chronic inflammation but no evidence of melanoma. This case highlights the utility of CEUS in delineating occult lesions on grayscale imaging, and its use in targeting biologically active portions of a lesion. Short interval follow-up MRI was stable, and the patient is currently presumed to be disease free.
Case No. 4: Hepatocellular Carcinoma from a Hepatic Adenoma
An adult with no significant medical history presented with an incidental liver mass noted on chest CT ( Figure 4 ). Further characterization with MRI revealed multiple hepatic lesions, the largest of which measured 10.8 × 7.0 cm, with mild T2 hyperintensity, heterogeneous arterial enhancement, and equivocal regions of washout. The lesions all displayed arterial enhancement without washout. A biopsy of the largest lesion revealed a well-differentiated hepatocellular carcinoma arising from within a hepatic adenoma. The patient underwent partial hepatectomy with biopsy and ablation of the remaining liver lesions greater than 1 cm. Pathology of the smaller lesions revealed multiple hepatic adenomas.
MRI demonstrates an enhancing mass at the inferior aspect of the right lobe of the liver (A, blue arrowhead), with pathology confirming hepatocellular carcinoma arising from an adenoma. Subsequently, lesions > 1 cm were ablated. ( B )Middle image demonstrates postablation changes from a treated adenoma (arrow). An enhancing lesion is present at the hepatic dome (arrowhead), consistent with an adenoma that did not meet the size criteria for ablation. ( C )On surveillance imaging, the adenoma appears hypoechoic on grayscale images (arrowhead) but is without any suspicious enhancement on contrast-enhaced US, as noted on short-term follow-up during pregnancy.
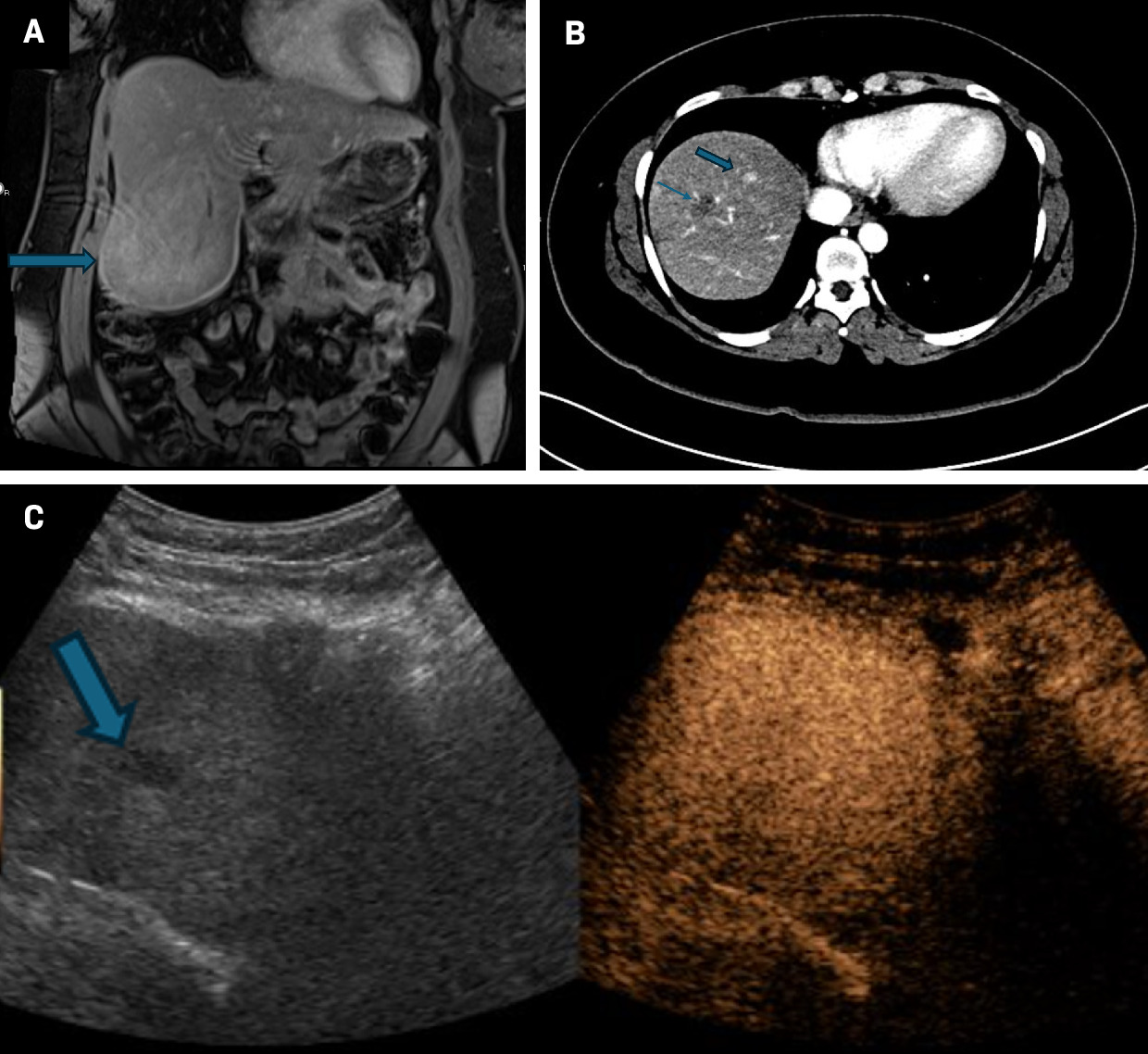
Following liver resection, the patient became pregnant. Owing to the risk of growth and rupture during pregnancy, the patient was monitored with serial imaging and alpha-fetoprotein. Since contrast-enhanced MRI is not recommended to monitor lesion size in pregnancy, CEUS was used to assess lesion stability for short-term follow-up. The patient’s most recent CEUS demonstrated mild growth of the largest hepatic adenoma but no suspicious washout, and continued surveillance was recommended.
Discussion
The liver is one of the most common sites of metastatic disease. In general, the prognosis for patients with hepatic metastases has historically been poor.24 In the past decade, however, effective systemic treatments have emerged for advanced metastatic disease, most notably immunotherapeutics.1 In this context, the frequency of surveillance imaging and hepatic biopsy has significantly increased.5, 8
CT is the mainstay of cancer imaging surveillance, often performed at intervals of 3-12 months.9 - 13 Imaging surveillance is typically accompanied by clinical follow-up and, when available, trending of serum tumor markers such as CEA (colorectal cancer), CA 19-9 (pancreatic cancer), and CA 125 (ovarian cancer).25 In certain malignancies, advanced imaging such as MRI, FDG-PET, or targeted radionuclide SPECT may also be performed.14
The response to a newly detected hepatic lesion is influenced by radiologic findings and clinical factors. Depending on the lesion’s appearance and chronicity, one can speculate on the likelihood of metastatic disease. Indeterminate findings may prompt consideration of biopsy for definitive diagnosis.18, 19 In cases of presumed tumor, the proliferation of novel targeted therapeutic options has increased the need for tissue sampling as many of these treatments are contingent on detection of specific targets requiring advanced histologic and immunohistochemical staining.1
This is particularly true in clinical trials, where therapies pose significant risks, and only a subset of patients with specific mutations may be eligible for enrollment.26 Alternatively, in certain cases, hepatic metastases may trigger re-evaluation of treatment options and objectives. For example, a new hepatic metastasis may indicate disease progression, resulting in changes to treatment regimen and imaging frequency.4 In cases of oligometastatic disease, percutaneous ablation or surgical wedge resection may be considered. Finally, instances where systemic and surgical treatment options are limited may prompt reassessment of care and disease management goals.
CT and US are the mainstays of image-guided intervention. US is typically preferred owing to the absence of ionizing radiation and the availability of continuous, real-time imaging.18 In cases of sonographically occult lesions, CECT has often been used as a guidance modality; however, many grayscale occult lesions can be effectively characterized and targeted with CEUS, obviating the need for ionizing radiation and potentially nephrotoxic iodinated contrast.19 - 22 In addition, multiple rounds of CEUS can be administered, allowing for initial image acquisition for diagnosis and subsequent real-time biopsy guidance.19 - 21 While contrast quickly degrades (typically within 10 minutes), microspheres can also be annihilated at any time using a high-powered pulse to shorten procedure length.
During a typical CEUS biopsy, a lesion is continuously imaged for at least 60 seconds, allowing visualization of sonographically occult lesions and assessment of lesion enhancement kinetics.23 In the setting of heterogeneous lesions, specific targets can be selected, not only to avoid nonenhancing areas of necrosis/debris but also to identify areas of avid arterial enhancement and washout predictive of more aggressive disease.21 CEUS is also shown to increase the accuracy of percutaneous liver biopsy, particularly in lesions < 2 cm, and reduce the number of puncture attempts.
This targeting is of growing importance as the use of certain immunotherapies depends not only on the presence but also the relative expression of specific tumor markers.27 As oncologic therapies progress, moreover, the need for representative tumor samples and CEUS for effective lesion characterization and sampling is likely to advance along with them.
References
Citation
Schick J, Mehta T, Sedaghat F.Contrast-Enhanced US for Characterization and Biopsy of Indeterminate Hepatic Lesions and Metastases: A Review with Case Examples. Appl Radiol. 2025; (Suppl_1):45-51.
doi:10.37549/AR-D-24-0066
February 14, 2025