Cerebral Venous Sinus Thrombosis
Case Summary
A teenager with a surgical history of craniotomy for craniosynostosis and a medical history of menorrhagia requiring oral contraceptives presented with 1 month of worsening frontal, parietotemporal, and occipital headaches. Initially, the headaches occurred only in the morning but progressed to occurring throughout the day. The patient noted that the headaches improved while lying flat and worsened with Valsalva, increased activity, and bending over.
Imaging Findings
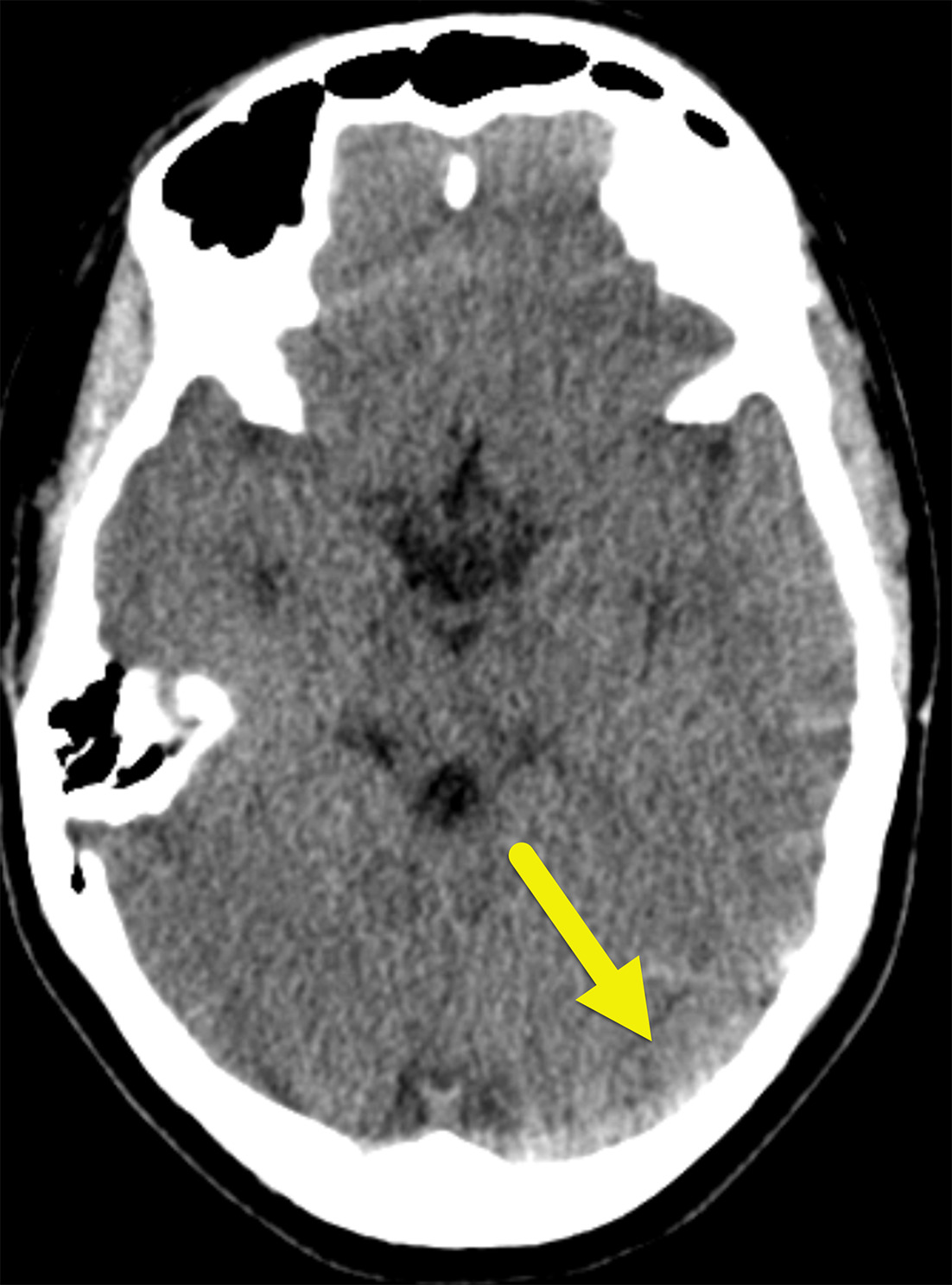
Noncontrast head CT ( Figure 1 ) showed asymmetric, increased attenuation in the left transverse and sigmoid sinuses extending to the superior aspect of the left internal jugular vein.
Noncontrast head CT showing asymmetric, increased density in the left transverse sinus (arrow).
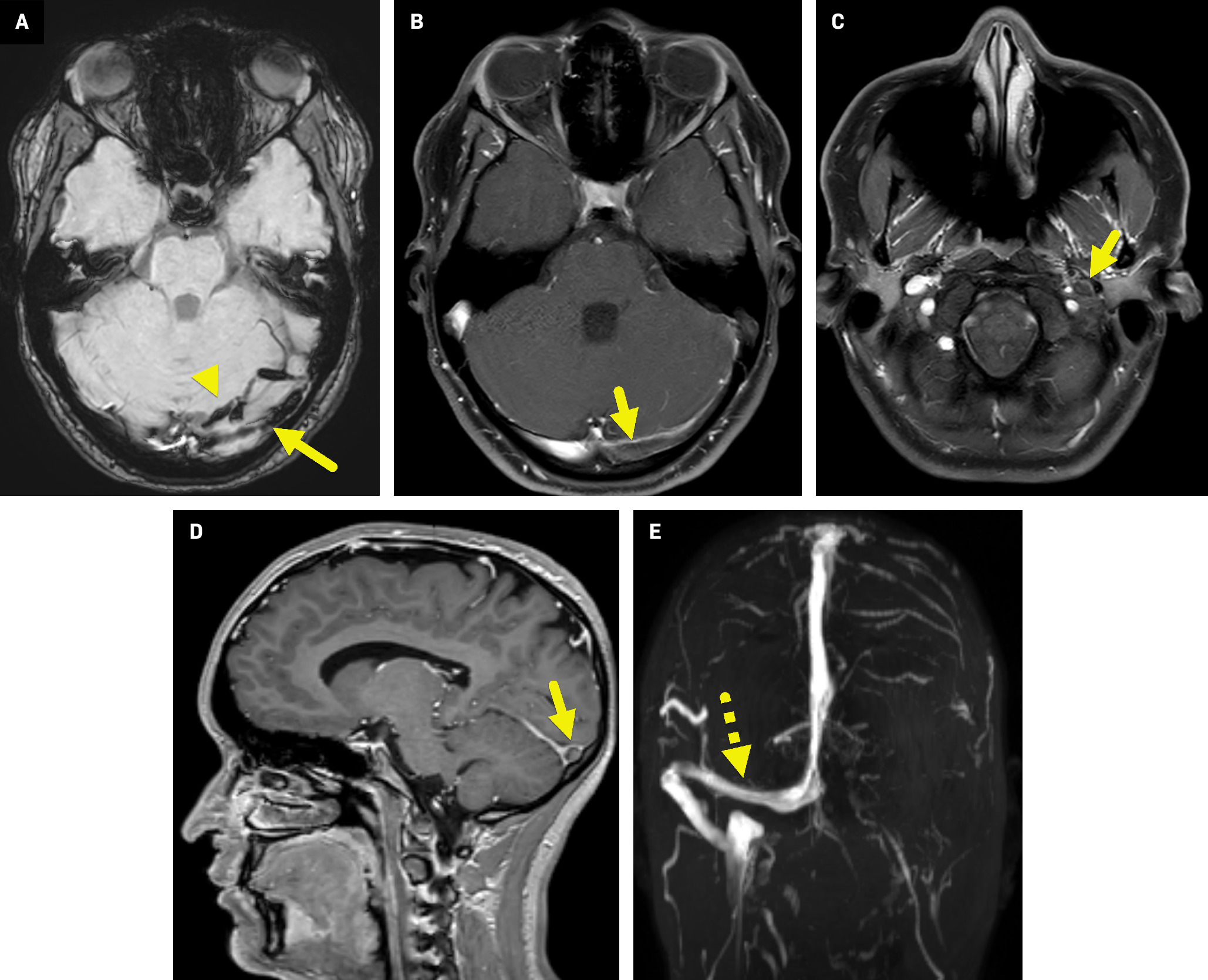
Subsequent MRI and magnetic resonance venography (MRV) of the brain ( Figure 2 ) confirmed the thrombus within the left transverse and sigmoid sinuses extending from the origin of the left internal jugular vein to the level of C5. There was a corresponding T1 hyperintensity, minimal T2 hyperintensity, and moderate magnetic susceptibility effect in these regions, consistent with an intraluminal thrombus.
Axial susceptibility-weighted image (A) shows moderate magnetic susceptibility effect in the left transverse sinus (arrow). There is also magnetic susceptibility effect in veins along the left side of the tentorium (arrowhead). Axial T1-weighted postcontrast fat-suppressed images at the level of the transverse sinus (B) and foramen magnum (C) show an intraluminal filling defect (arrow) within the left transverse sinus extending to the left internal jugular vein (C). In the sagittal T1-gradient echo postcontrast image ( D ), the intraluminal filling defect is surrounded by contrast, giving a form of the “empty delta sign.” Coronal maximum intensity slab image ( E ) from the time of flight MRV highlights normal flow-related enhancement in the right transverse sinus (dashed arrow) extending to the right internal jugular vein. No flow-related enhancement is seen on the left owing to the sinus venous thrombosis.
Diagnosis
Cerebral venous sinus thrombosis (CVST).
The differential diagnosis for new-onset headaches includes primary and secondary causes. Primary causes include tension, migraine, and cluster headaches.1 Secondary headaches can stem from intracranial tumors, hemorrhage, venous sinus thrombosis, increased intracranial pressure, and trauma.2
Discussion
In adults, the annual incidence of CVST is estimated to be 5 cases per million.3 It is more common in women.3 This differs from children, where the annual incidence is approximately 7 cases per million.4 Pediatric CVST occurs most commonly in neonates. There is no gender difference in incidence in children.4
CVST may occur because of local or systemic factors. Local factors that impact venous sinus blood flow include trauma, infection, and neoplasm. Systemic factors include medications such as oral contraceptives and prothrombotic conditions or states. CVST has no known cause in up to 25% of patients.5
Headache is the most common presenting symptom in up to 95% of patients.5 Other nonspecific signs and symptoms may include lethargy, mental status changes, and vomiting. Focal neurological deficits can also occur. These are more common in patients with parenchymal changes on imaging.5
In CVST, the increased venous pressure and subsequent disruption of the blood-brain barrier lead to extravasation of fluid, causing cerebral edema.6 If the elevated venous pressure exceeds arterial pressure, ischemia or hemorrhagic stroke can occur.6 Depending on its location, the thrombus can prevent reabsorption of cerebrospinal fluid from the periventricular regions.6
The superficial venous system is most commonly affected, with the superior sagittal and transverse sinuses serving as primary locations. The internal jugular and cerebral veins are also common sites of thrombosis.6
Diagnosis is made via imaging, and the initial imaging modality of choice depends on the population. In neonates, transfontanelle US is often the first-line imaging technique used due to its noninvasive nature and bed-side portability.7 This is usually followed by brain CT or MRI to determine the extent of the thrombus and its impact. In children and adults, noncontrast head CT is typically the initial imaging modality. If the results are positive or equivocal with high clinical suspicion, a CT venogram or MRI/MRV is often obtained to determine the extent and impact of the thrombosis.7
CVST may be diagnosed by the “cord” sign or “empty delta” sign. The cord sign is used to describe the linear, expanded, hyperdense venous sinus on noncontrast CT. It is not a sensitive sign as it is only present in ~25%-56% of cases.8 The empty delta sign occurs on postcontrast CT or MRI when the thrombus appears as a triangular-shaped filling defect surrounded by enhancing dural venous sinus or collateral channels, typically on axial imaging.5 This sign is only present in approximately 29% to 35% of patients.8
On MRI, CVST may be first diagnosed with an absent or altered flow void.8 The thrombus varies in signal intensity depending on its age. An acute CVST (1-5 d) appears isointense on T1-weighted images and hypointense on T2-weighted images, a subacute CVST (6-15 d) appears as hyperintense on both T1- and T2-weighted images, and a chronic CVST (>15 d) is isointense on T1-weighted images and iso/hyperintense on T2-weighted images.8 MRI can also detect associated findings such as sinusitis, emypema, brain parenchymal abnormalities near the site of thrombus (~40%-60%), parenchymal hemorrhages (~30%-35%), and — less commonly — gyral edema/enhancement, diffuse edema, and decreased size of ventricles secondary to mass effect.8
The treatment for CVST is typically divided into acute phase and chronic phase. During the acute phase, treatment depends on the condition’s severity. Patients with new focal neurological deficits should be considered for thrombolysis or endovascular thrombectomy if feasible.8 Both acute and chronic phases warrant anticoagulation.8
Conclusion
CVST is a rare disorder more common in the pediatric population, especially in neonates. Presenting signs and symptoms are nonspecific and include headache, lethargy, altered mentation, vomiting, or focal neurological deficits. Imaging is crucial for diagnosing and determining CVST location. Treatment typically involves anticoagulation, thrombolysis, and/or endovascular thrombectomy.
References
Citation
Yahia RW, Towbin RB, Schaefer CM, Morgan D, Towbin AJ.Cerebral Venous Sinus Thrombosis. Appl Radiol. 2024; (5):1 - 4.
doi:10.37549/AR-D-24-0016
August 1, 2024