Cerebral Abscess Secondary to Streptococcus mitis Infective Endocarditis
Images
CASE SUMMARY
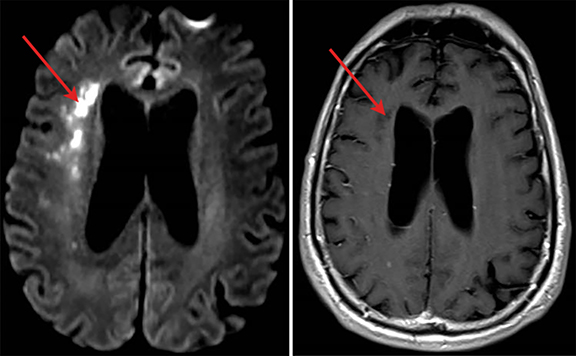
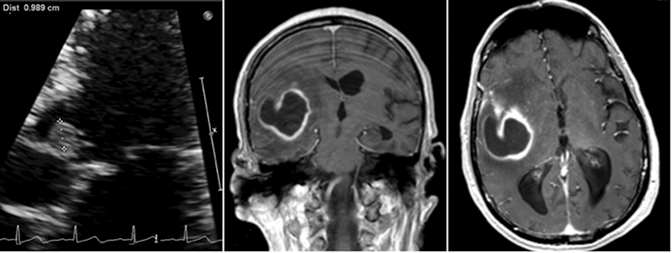
A 70-year-old man recently hospitalized for a right middle cerebral artery stroke noted on MRI of the brain (Figure 1) presented two days after discharge with altered mental status. The cerebrovascular accident from prior admission was thought to be cardio-embolic in nature; however, no arrhythmias were noted and transesophageal echocardiogram demonstrated no findings of vegetation or PFO. Upon re-admission, the patient was febrile with widened pulse pressure of 130/48. Physical exam was notable for a low-pitched early diastolic murmur. No other stigmata of infective endocarditis (IE) were found. Labs were significant for a leukocytosis of 21.8 (86% neutrophils). Brain computerized tomography (CT) demonstrated a lesion near the right Sylvian fissure with displacement of the septum pellucidum. Subsequent bedside transthoracic echocardiogram was notable for aortic valve vegetation (Figure 2) as well as severe aortic regurgitation. Stat MRI of the brain was ordered to further delineate the brain lesion prior to surgical intervention.
IMAGING FINDINGS
Axial and coronal (Figure 2) views on T1 post-contrast flash MRI of the brain demonstrated a hyperintense thick-walled enhancing lesion in the right temporal lobe measuring 3.6 × 3.5 × 3.3 cm in transverse, AP, and vertical dimensions. Surrounding extensive right frontal temporal parietal vasogenic edema was noted with associated mass effect, 6 mm of midline shift and developing uncal herniation. Upon retrospective review, the previously diagnosed stroke was likely the early findings of a developing cerebral abscess secondary to embolic fragments from IE.
DIAGNOSIS
Cerebral abscess secondary to Streptococcus mitis infective endocarditis
DISCUSSION
Systemic emboli are estimated to occur in 20-50% of IE patients, with the central nervous system being the most common location affected by dislodged valvular vegetations.1 However, cerebral abscesses are a rare complication of these emboli seen in only 1-7% of cases.2 In such situations, the timing of cardiothoracic and neurosurgical interventions can be a difficult decision for physicians.
Management of cerebral abscesses secondary to IE should occur in a stepwise and decisive manner once diagnosis is made with CT of the brain. Initial treatment with dexamethasone is indicated for patients with neurologic findings on exam or substantial mass effect on imaging.3 Dexamethasone is thought to result in decreased inflammation, cerebral edema, intracranial pressure, and risk of herniation. Patients should also receive a loading dose of Keppra, followed by twice-daily maintenance therapy to prevent seizures, which are associated with 30-60% of cases of cerebral abscess.4 Blood culture results and sensitivities should be used to guide initiation of appropriate antibiotic administration. In patients where a causative organism has not been isolated, an empiric regimen of vancomycin, ceftriaxone or cefotaxime, and metronidazole is recommended.5
The next step in management entails a multidisciplinary approach that requires input from cardiothoracic, neurosurgical, and infectious disease specialists. Urgent valve replacement should be considered in patients with hemodynamic instability, severe heart failure, perivalvular abscess, prosthetic valve infection, heart block, ongoing recurrent emboli despite treatment with antibiotics, or vegetations greater than 10 mm.6 Hemodynamically stable patients with none of the previously listed comorbidities should be evaluated for neurosurgery prior to valve replacement.7
While intuitively the source of the problem (infected valve) should be dealt with prior to the cerebral abscess, valve replacement surgery entails the use of cardiopulmonary bypass and systemic heparinization subsequently increasing the risk of hemorrhagic conversion. Cardiopulmonary bypass also increases the risk of hypotension which can exacerbate the preexisting neurologic complication and result in worsening cerebral edema. Of note, early valve replacement may increase the risk for infection of the prosthetic valve due to inadequate bacterial clearance. Consequently, we recommend neurosurgery as the initial treatment modality in this subset of patients. While both needle aspiration and surgical excision have been used in the past to treat brain abscesses, the former is generally preferred for speech areas and regions of the sensory and motor cortex due to better outcomes.8 Following surgery, patients should continue antibiotics for 4-8 weeks with timing of subsequent valve replacement within 2-4 weeks following neurosurgical intervention.
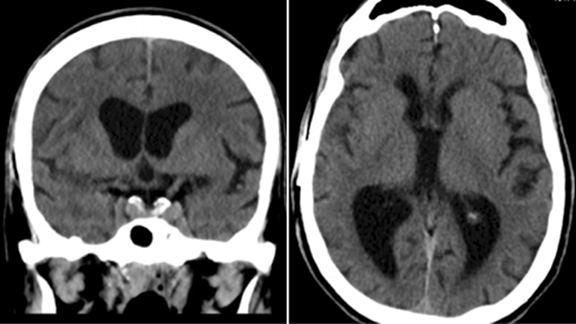
Our patient was started on dexamethasone for cerebral edema, Keppra for seizure prophylaxis, and an empiric antibiotic regimen. Aspirin was held and the patient was taken for needle aspiration. The patient demonstrated clinical improvement following the procedure and aortic valve replacement surgery was undertaken 3 weeks later. Blood and brain abscess cultures came back positive for Strep mitis, likely secondary to poor dentition. The patient made a full recovery with no residual neurologic or cardiac deficits. A CT scan of the brain was repeated 3 months following the previous MRI; the CT scan demonstrated resolution of the brain abscess, cerebral edema, and midline shift (Figure 3).
CONCLUSION
While management of IE with cerebrovascular complications should be addressed on a case-by-case multidisciplinary assessment of potential risks and benefits of intracranial and cardiac operations, we recommend that cerebral abscesses should always be addressed first in hemodynamically stable patients. The need for cardiopulmonary bypass and subsequently systemic heparinization with valve replacement surgery is a key factor in this decision as hemorrhagic conversion is a potential complication. The treatment strategy reflected in the above discussion is essential for physicians encountering similar clinical scenarios.
REFERENCES
- Habib G, et al. 2015 ESC Guidelines for the management of infective endocarditis: The task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European Heart Journal 36.44(2015): 3075–3128.
- Morris NA, et al. Neurologic complications in infective endocarditis: Identification, management, and impact on cardiac surgery. The Neurohospitalist 4.4(2014): 213–222.
- Quartey J, et al. Decadron in the treatment of cerebral abscess. An experimental study. J. Neurosurg. 45.3(1976): 301–310.
- Seydoux C, et al. Bacterial brain abscesses: factors influencing mortality and sequelae. Clin. Infect. Dis. 15.3(1992): 394–401.
- Baddour L, et al. Infective Endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 132.15(2015): 1435.
- Angstwurm K, et al. Timing the valve replacement in infective endocarditis involving the brain. J Neurol. 2004; 251(10): 1220-1226.
- Molnar A, et al. Drainage of cerebral abscesses prior to valve replacement in stable patients with acute left-sided infective endocarditis. CNS & Neurological Disorders – Drug Targets. 14.4(2015): 534-539.
- Ratnaike T, et al. A review of brain abscess surgical treatment–78 years: aspiration versus excision. World Neurosurg. 76.5(2011): 431–436.
Citation
M L, A S, M A, I D, D W.Cerebral Abscess Secondary to Streptococcus mitis Infective Endocarditis. Appl Radiol. 2020; (2):45-47.
March 17, 2020