Metastatic endometrial serous carcinoma to the breast
By Lin T, Lin D, Zhang Z, Shah BA


CASE SUMMARY
A 74-year-old post-menopausal woman presents with vaginal bleeding for several months. Endometrial curettage and subsequent histopathology arrived at the diagnosis of endometrial serous carcinoma. Shortly after, the patient developed a lump in her right breast. Mammogram of the palpable anomaly revealed a suspicious mass that was later biopsied using ultrasound guidance. Histopathology revealed metastatic endometrial serous carcinoma.
IMAGING FINDINGS
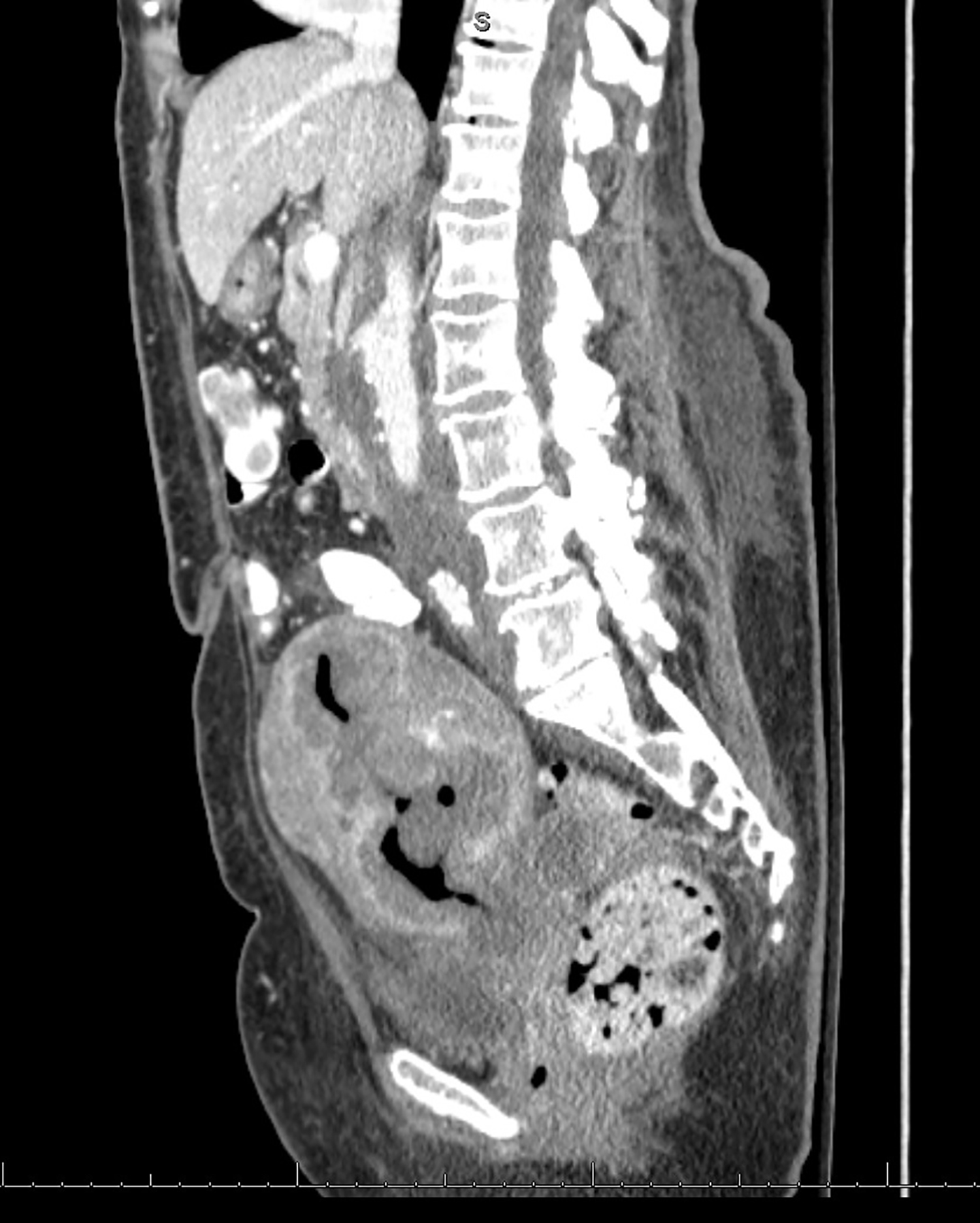
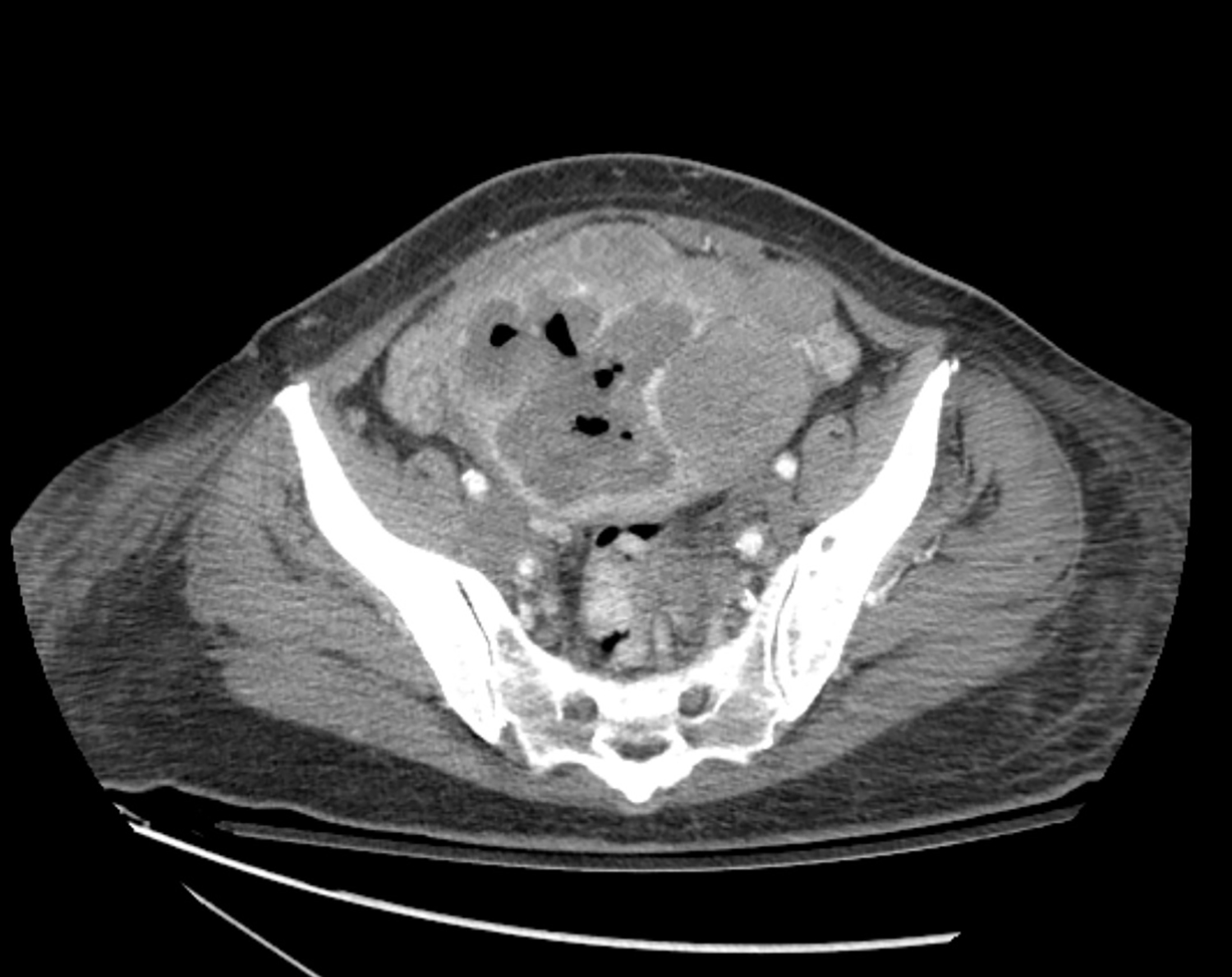
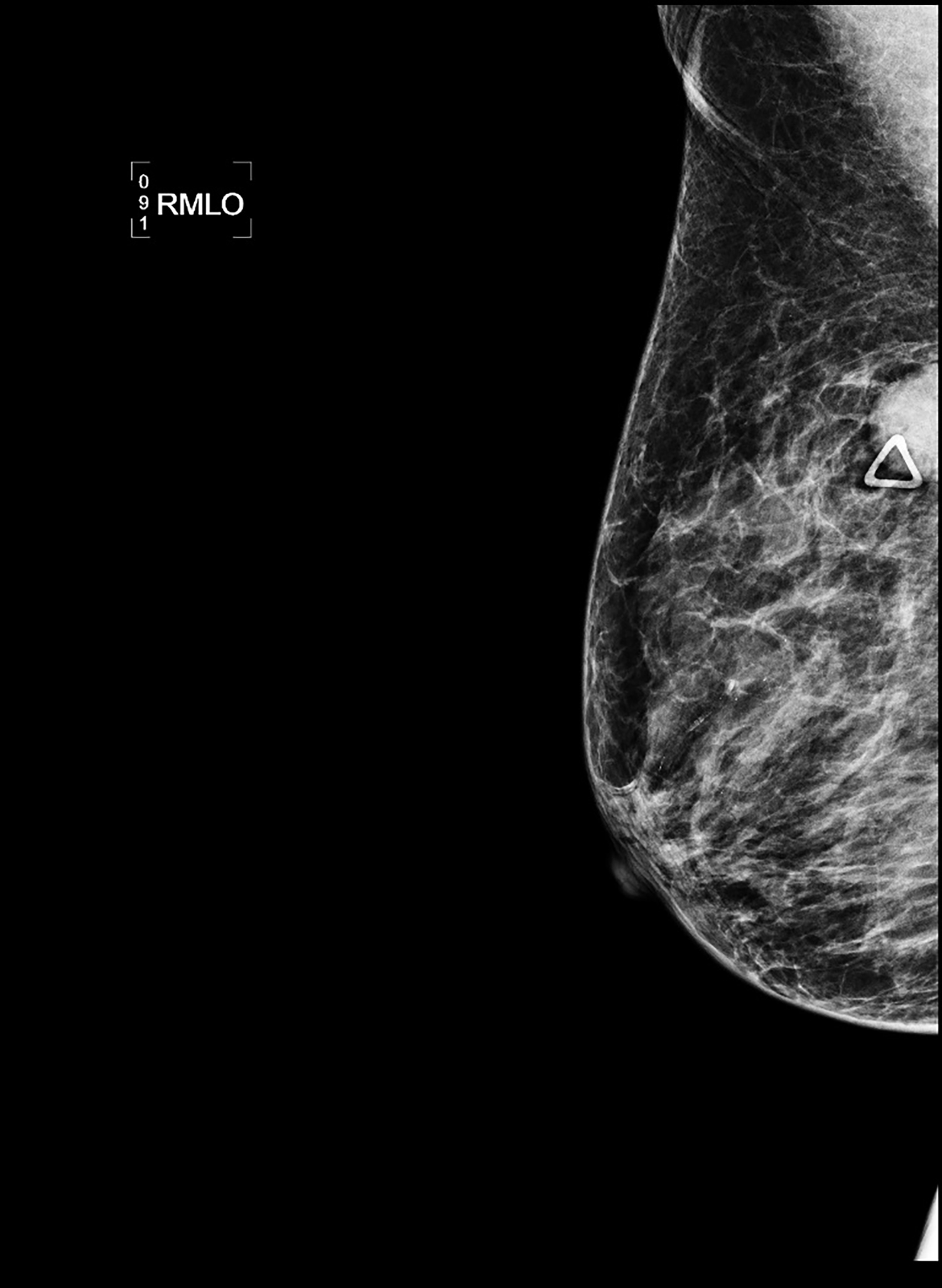
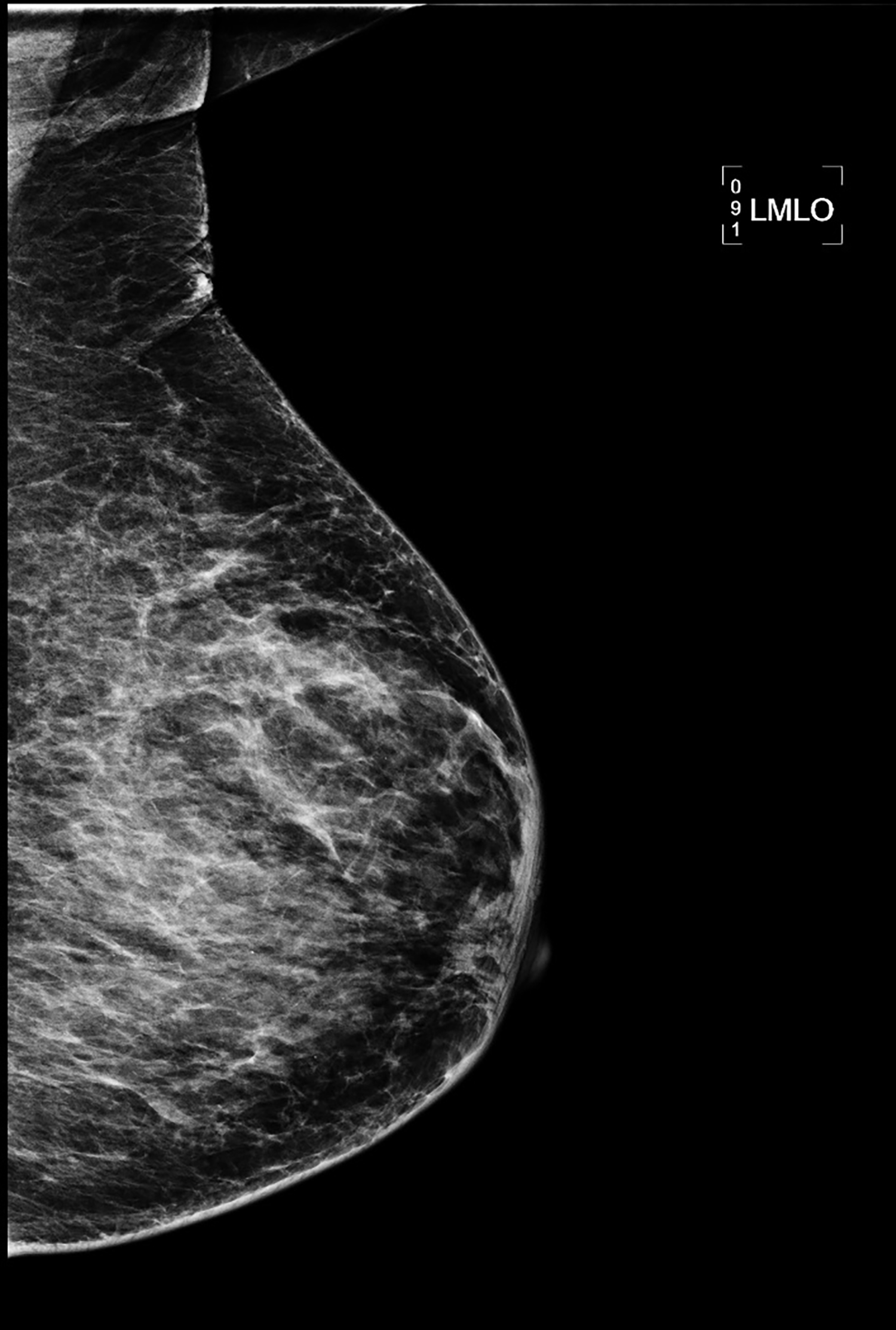
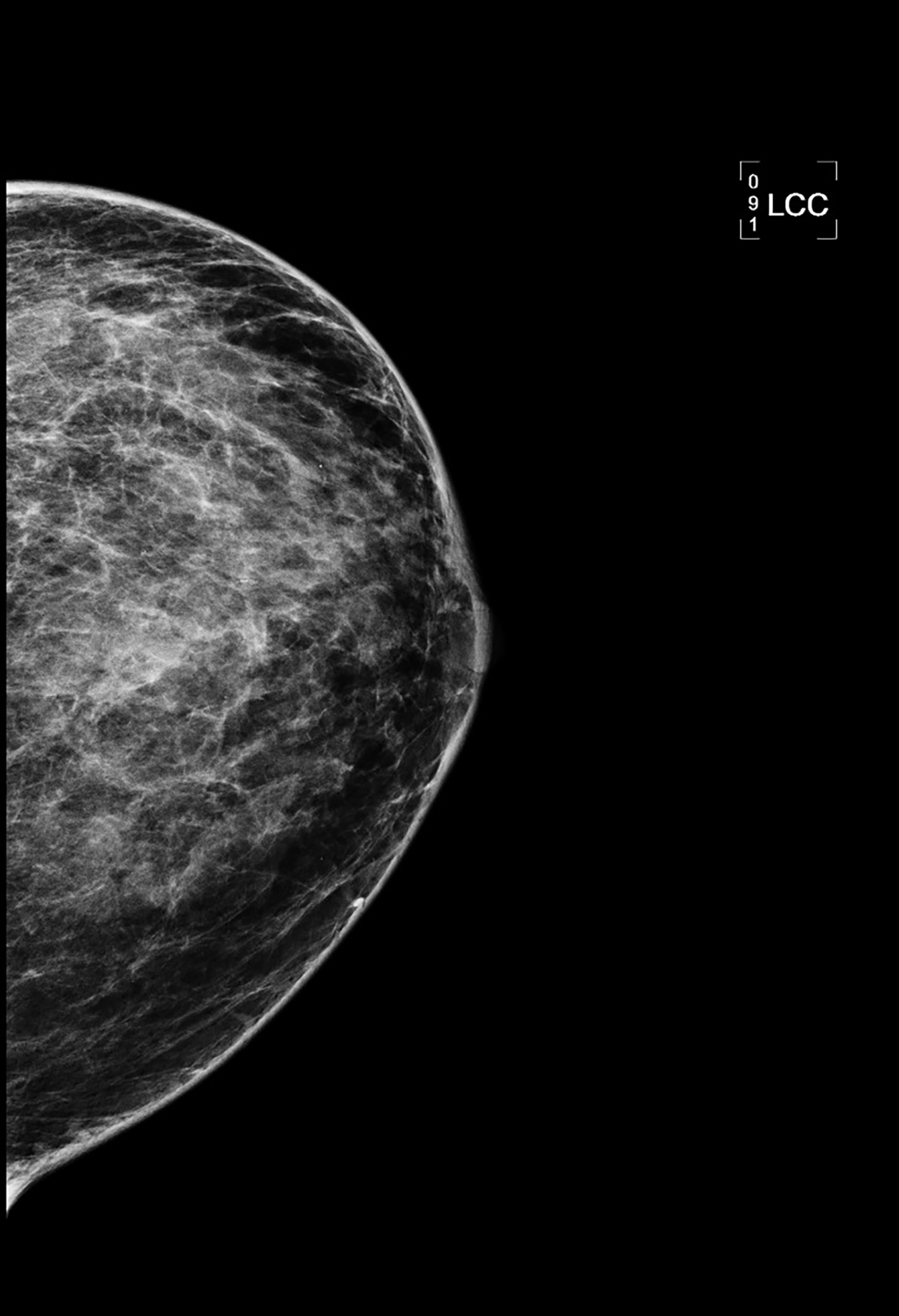
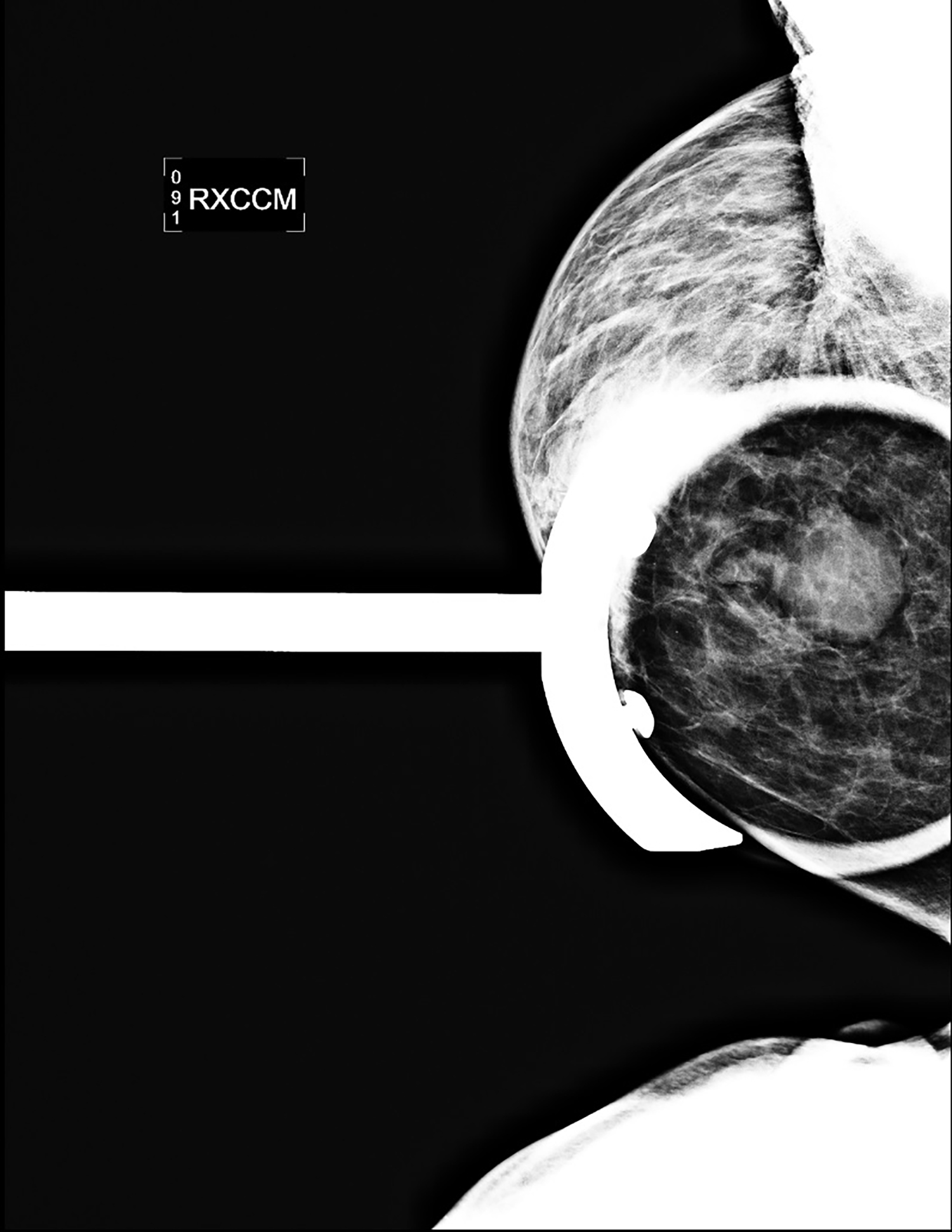
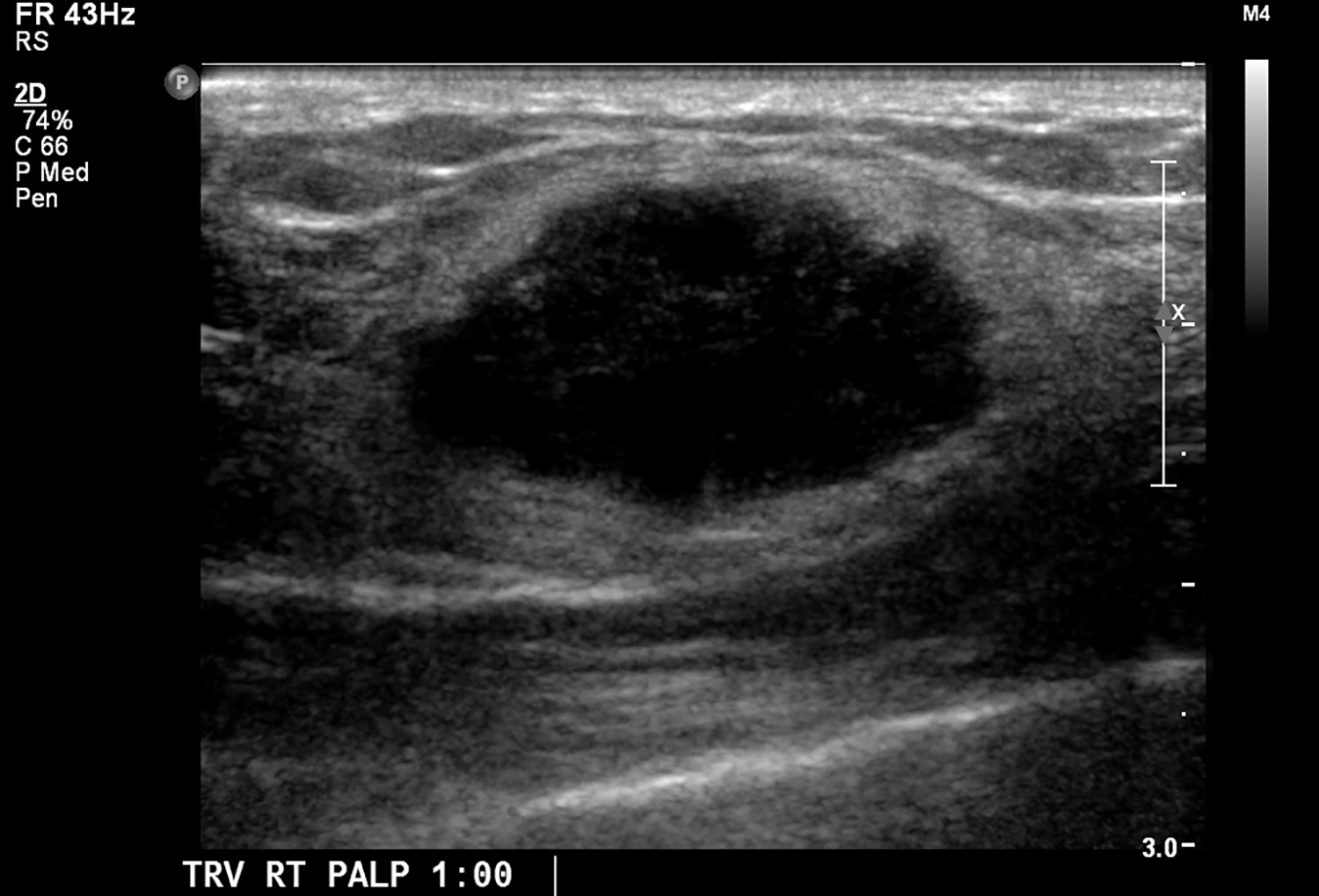
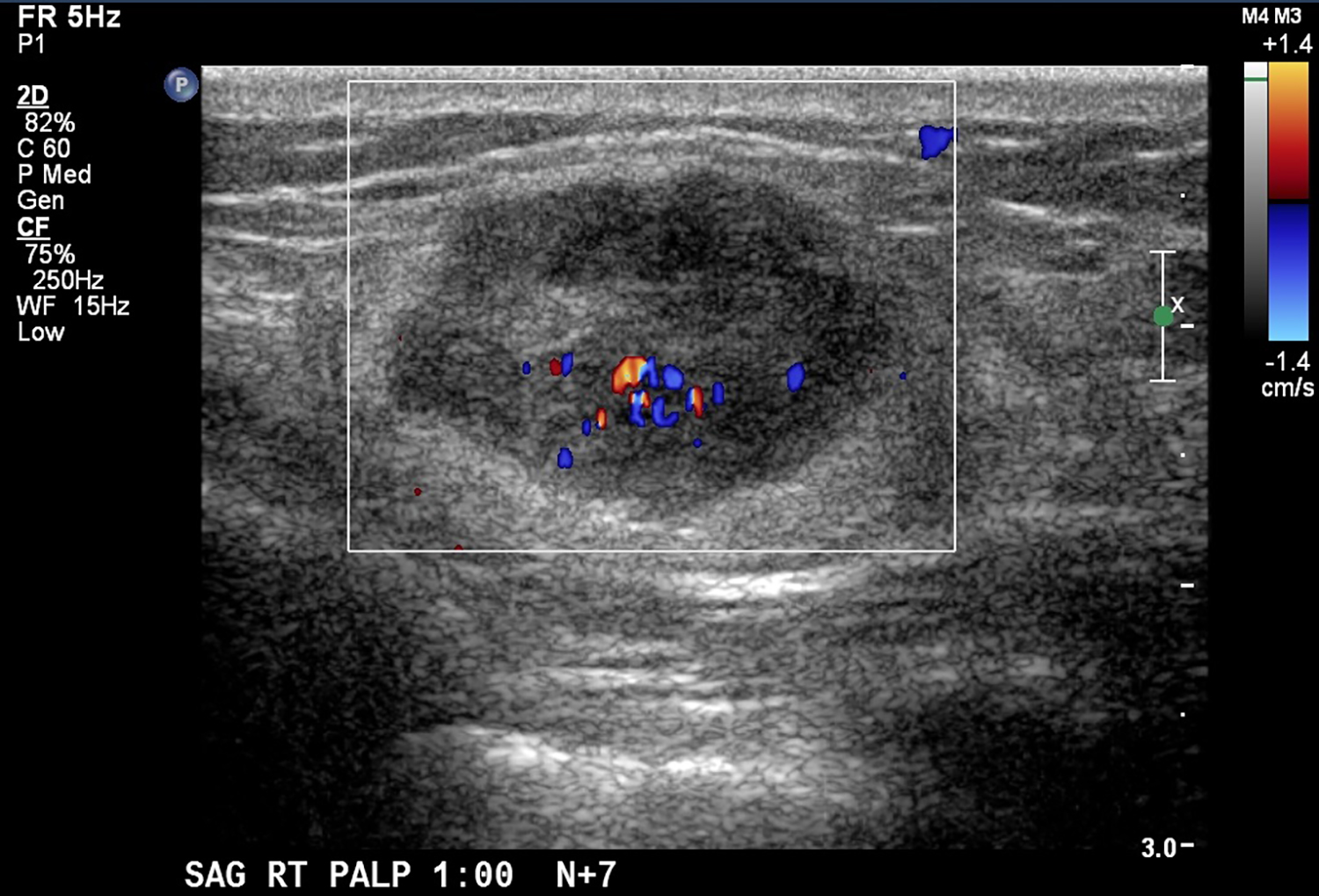
CT with intravenous and oral contrast revealed a markedly enlarged uterus in concordance with the diagnosis of endometrial cancer (Figure 1). Shifting attention to the suspicious breast lesion, diagnostic mammogram revealed a round mass at the site of the palpable abnormality (Figure 2) and targeted ultrasound demonstrated a round hypoechoic mass with mild internal vascularity (Figure 3).
DIAGNOSIS
Metastatic endometrial serous carcinoma to the breast
DISCUSSION
Endometrial carcinoma is a common gynecologic malignancy that typically affects post-menopausal women during their 6th or 7th decade of life. Clinical presentation of endometrial cancer may consist of abnormal vaginal bleeding, pelvic pain, and involuntary weight loss. Subtypes of endometrial cancer include the more common type I, endometrioid adenocarcinoma, and the rare type II, which encompasses clear-cell, carcinosarcomas, and endometrial serous carcinoma.1 All type II endometrial cancers are high-grade tumors. Definitive diagnosis involves dilation and curettage or endometrial biopsy.
Endometrial carcinoma may spread locally by direct infiltration as well as systemically via lymphatic or hematogenous routes. Endometrial cancer staging utilizes surgicopathologic findings from exploratory laparotomy, abdominal hysterectomy, bilateral salpingo-oophorectomy, peritoneal lavage, and pelvic and para-aortic lymphadenectomy.1 Preoperative imaging using transvaginal ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), and more recently, positron emission tomography (PET), offer the benefit of identifying distal metastases. Typical metastatic sites include local pelvic recurrence, abdominal lymph nodes, peritoneum, and lungs.2 Rare metastatic targets include extra-abdominal lymph nodes, liver, adrenal glands, brain, bones, and soft tissues.
The breast is a rare target of extramammary metastases, with the most common primary tumors being melanomas, leukemias, or lymphomas.3 Endometrial metastases to the breast are even more rare. To our best knowledge, there is only one other documented account of metastatic endometrial serous carcinoma to the breast.4 Rates of clinically observed extramammary metastases to the breast range from 0.5 to 1.3%.5 However, this frequency is low compared to autopsy studies that reported 6.6% of breast tumors to be non-primary.6 Discrepancy in the aforementioned findings could indicate that metastases to the breast may occur later during advanced malignant disease or with rare high-grade tumors such as type II endometrial cancer.
Metastatic disease to the breast may mimic primary breast cancer on imaging studies. Sonographically, extramammary metastases may appear differently when the cancer seeds the breast through hematogenous versus lymphatic routes.7 Hematogenous metastases tend to colonize richly vascularized areas of the breast. Typical US presentation includes single or multiple circumscribed hypoechoic oval masses involving only one breast. Features common to primary breast cancer such as spiculations, calcification, parenchymal distortion, acoustic shadowing, secondary skin or nipple changes are typically not observed with hematogenous metastases to the breast. Sonographic appearance of lymphatic metastases may mimic inflammatory primary breast cancer by demonstrating heterogeneous echogenicity, coarse trabecular pattern, skin thickening and lymphedema. On mammography, the morphology of metastases to the breast is that of an oval circumscribed mass with no associated microcalcifications and no desmoplastic features.
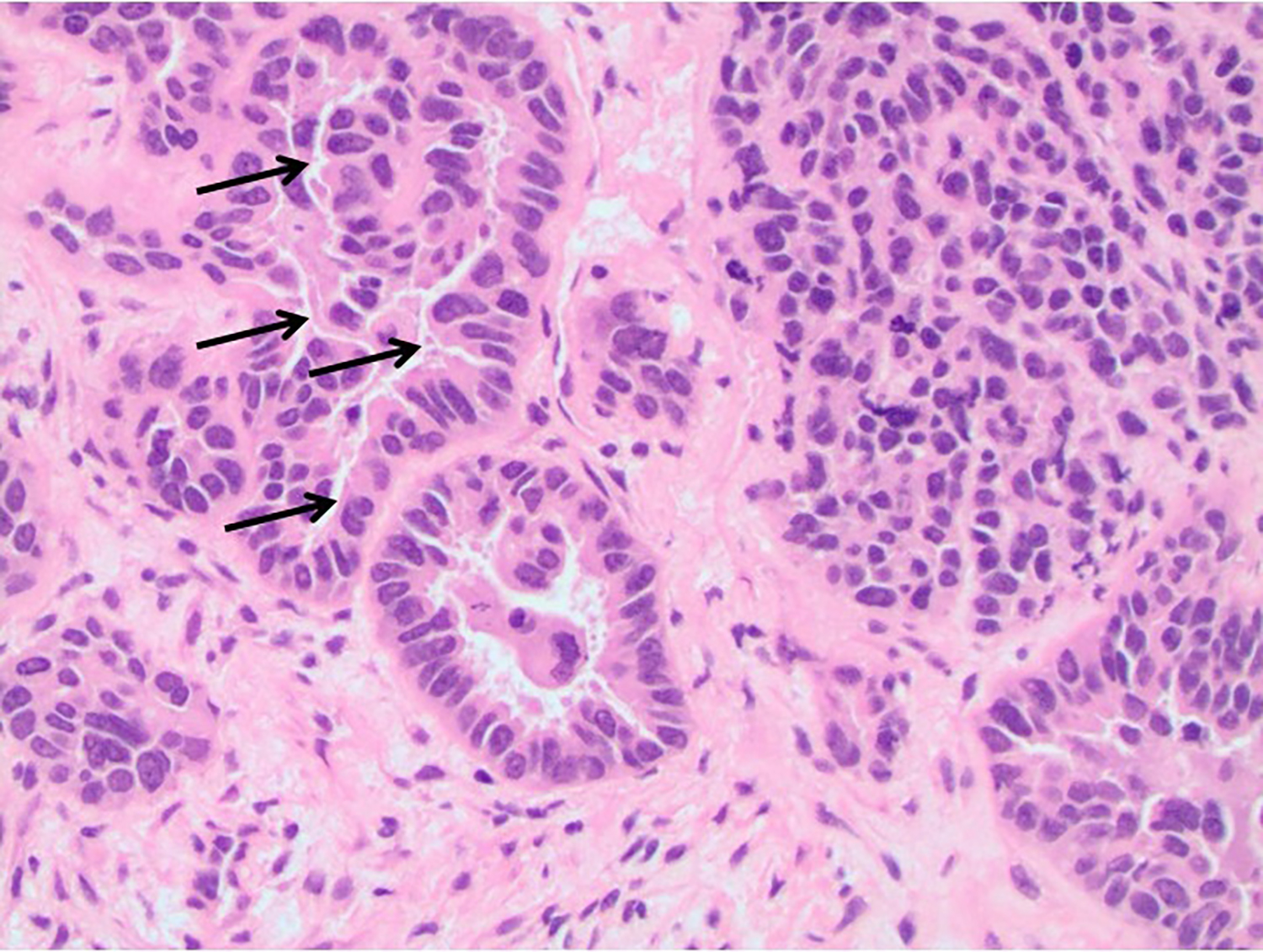
Diagnosis of metastases to the breast can be confirmed using comparative histopathology of the mammary lesion and the primary cancer. For primary endometrial serous carcinoma, common histological features from the endometrial biopsy include papillae containing highly pleomorphic tumor cells with glandular structure.8 Myometrial invasion and high mitotic activity are also frequently observed due to the cancer’s aggressive nature. Psammoma bodies may be present, but they are not distinguishing features of endometrial serous carcinoma.
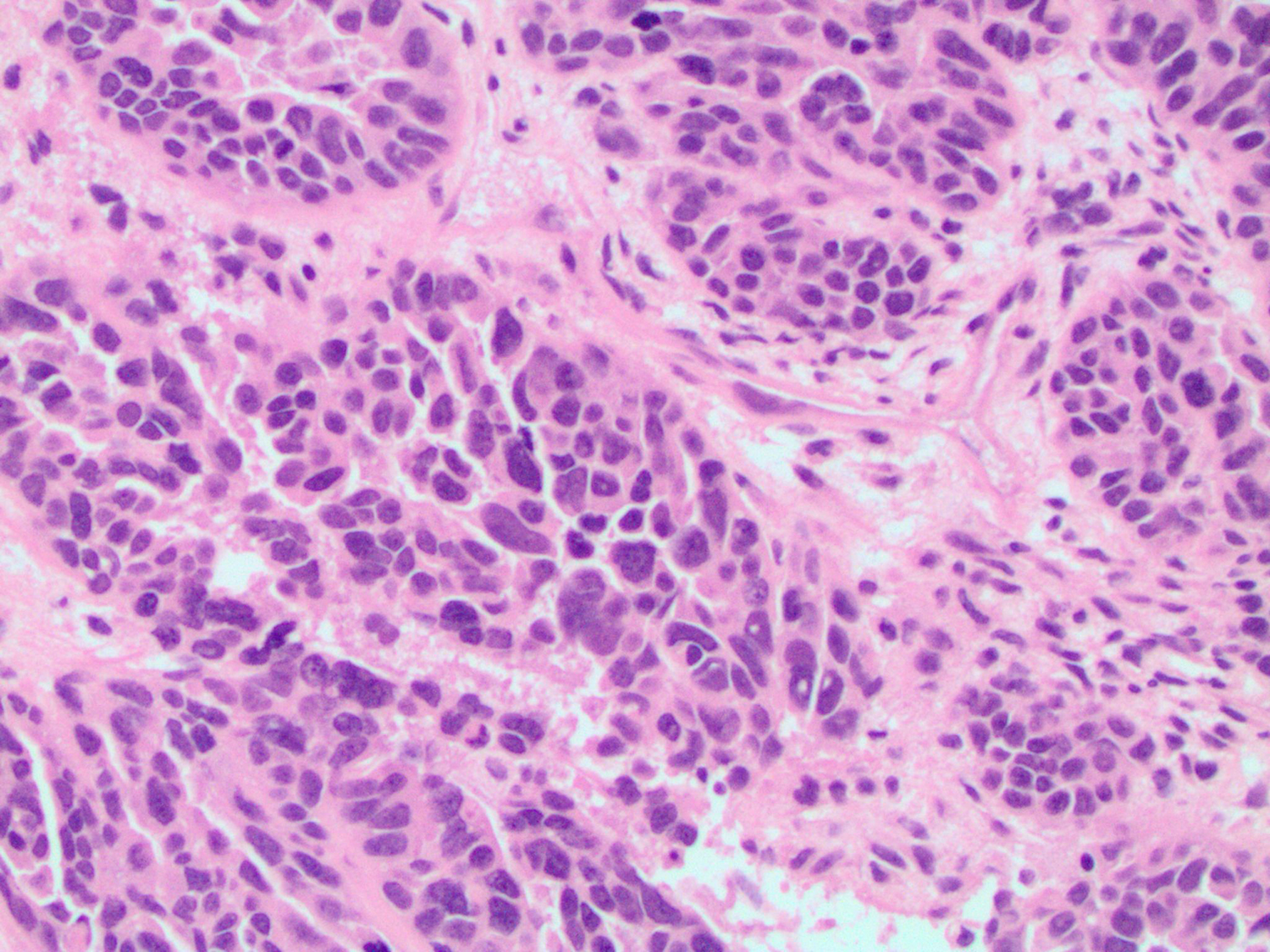
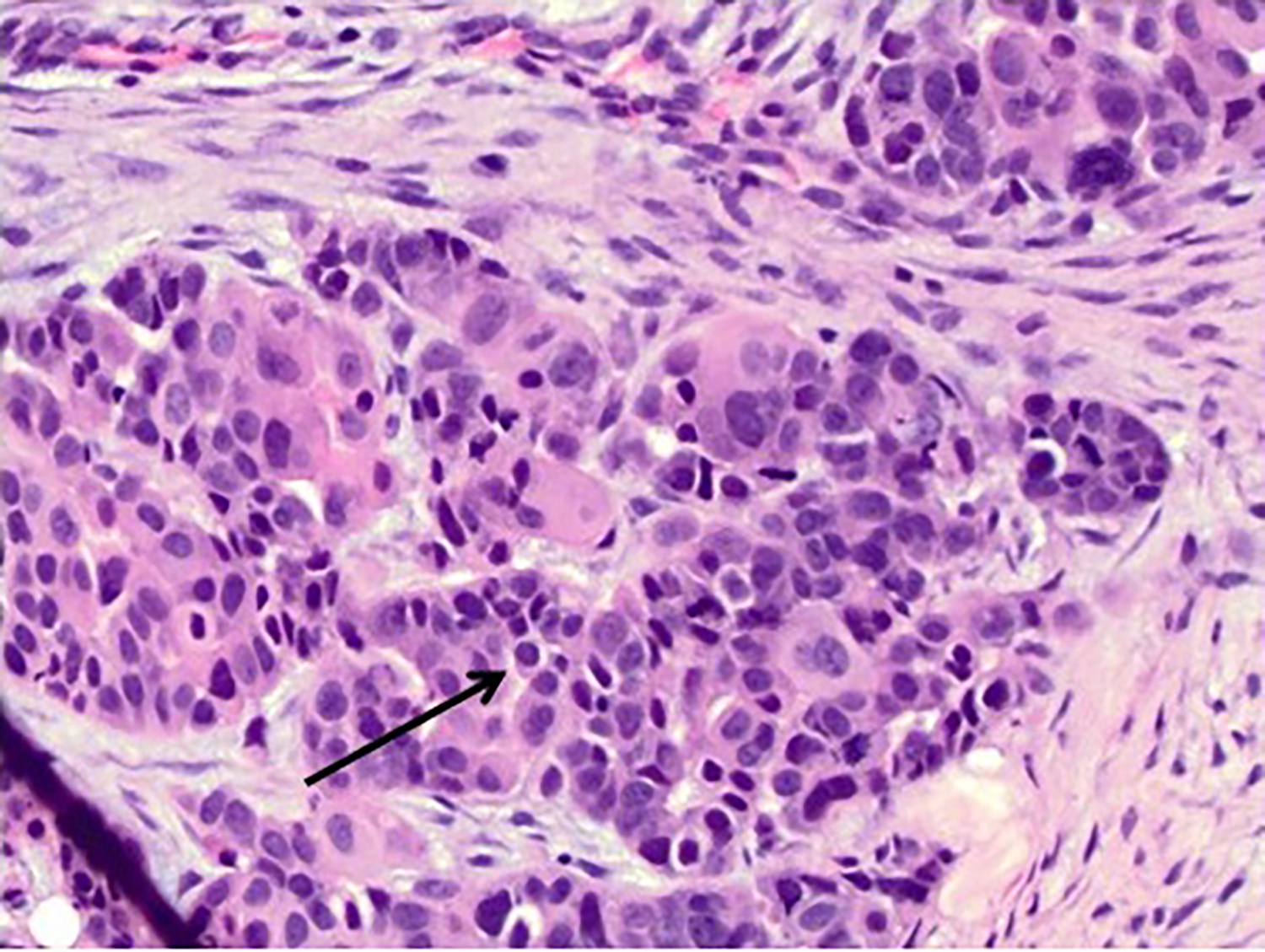
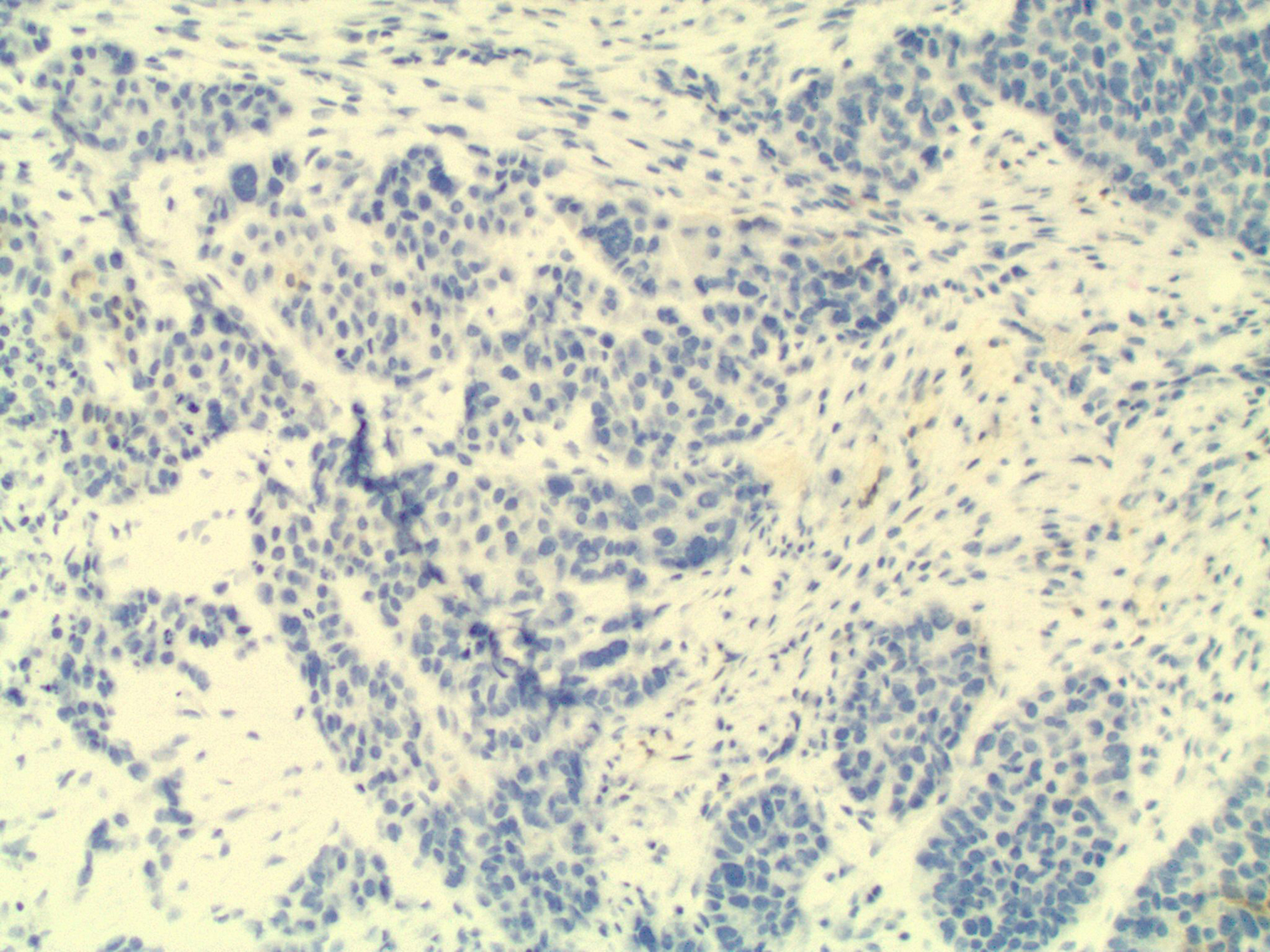
In this case study, histology of the endometrial curettage revealed pleomorphic glandular tumor cells consistent with endometrial serous carcinoma (Figure 4). Furthermore, ultrasound-guided core needle biopsy of the breast showed tumor cells with morphology and architecture similar to the endometrial curettage samples (Figure 5). Subsequent staining for breast carcinoma marker mammaglobin was negative and evidence of ductal carcinoma in situ was absent. All the evidence considered, the diagnosis for the suspicious breast lesion argued against primary breast carcinoma, but instead was more consistent with metastatic endometrial serous carcinoma to the breast.
CONCLUSION
Metastatic disease to the breast is an uncommon but not rare occurrence. Melanomas and hematopoietic malignancies that infiltrate the breast are well documented by several investigators. Conversely, metastatic endometrial serous carcinoma to the breast, as presented in this case study, is especially unique. In order to implement the appropriate treatment, it is important to distinguish metastatic from primary breast lesions. This can be best accomplished by being cognizant that the breast is an atypical, but possible, target of metastases. Breast metastases may resemble primary breast cancer under mammogram and ultrasound. However, they usually lack the microcalcifications and spiculations characteristic of primary breast cancer. Tissue biopsies of the suspicious breast lesion are crucial for the correct diagnosis that will subsequently guide disease management and treatment.
REFERENCES
- Faria SC, Sagebiel T, Balachandran A, Devine C, Lal C, Bhosale PR. Imaging in endometrial carcinoma. Indian J Radiol Imaging. 2015;25(2): 137-47.
- Kurra V, Krajewski KM, Jagannathan J, Giardino A, Berlin S, Ramaiya N. Typical and atypical metastatic sites of recurrent endometrial carcinoma. Cancer Imaging. 2013;13:113-22.
- Akçay MN. Metastatic disease in the breast. Breast. 2002;11(6):526-8.
- Fulciniti F, Losito S, Botti G, et al. Metastases to the breast: role of fine needle cytology samples. Our experience with nine cases in 2 years. Ann Oncol. 2008;19(4):682-7.
- Bohman LG, Bassett LW, Gold RH, Voet R. Breast metastases from extramammary malignancies. Radiology. 1982;144(2):309-12.
- Toombs BD, Kalisher L. Metastatic disease to the breast: clinical, pathologic, and radiographic features. AJR Am J Roentgenol. 1977;129(4): 673-676.
- Mun SH, Ko EY, Han BK, Shin JH, Kim SJ, Cho EY. Breast metastases from extramammary malignancies: typical and atypical ultrasound features. Korean J Radiol. 2014;15(1):20-8.
- Abeler VM, Kjørstad KE. Serous papillary carcinoma of the endometrium: a histopathological study of 22 cases. Gynecol Oncol. 1990;39(3):266-71.
Prepared by Tayson Lin, a first-year medical student at Wayne State School of Medicine in Detroit, MI, Dr. David Lin, a diagnostic radiology resident at Henry Ford Hospital in Detroit, MI, Dr. Ziying Zhang, a senior staff pathologist at Henry Ford Hospital in Detroit, MI, and Dr. Biren A. Shah, a senior staff radiologist at Henry Ford Hospital in Detroit, MI.
