A multimodality review of solid and cystic pancreatic masses
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Numerous solid and cystic pancreatic masses are encountered on cross-sectional imaging. Solid masses include pancreatic adenocarcinoma, neuroendocrine tumors, and metastases. Some masses, such as pancreatic adenocarcinoma and neuroendocrine tumors, are typically solid but uncommonly may be cystic. Cystic pancreatic masses include pseudocyst, serous cystadenoma, mucinous cystadenoma, intraductal papillary mucinous neoplasm, and solid pseudopapillary tumor. Diagnosis may be aided by a multimodality approach including multidetector CT, MRI, endoscopic ultrasound, single photon emission computed tomography (SPECT), and positron emission tomography (PET/CT).
Solid masses
Pancreatic adenocarcinoma
Pancreatic adenocarcinoma is an aggressive neoplasm representing approximately 85-95% of pancreatic malignancies. Most patients are 60-80 years of age, with males affected twice as often as females. Approximately 60-70% of pancreatic adenocarcinomas are located in the pancreatic head. Patients may present with abdominal pain, weight loss, or jaundice. Surgery is the only potential cure, but unfortunately 75% of patients present with unresectable disease.1
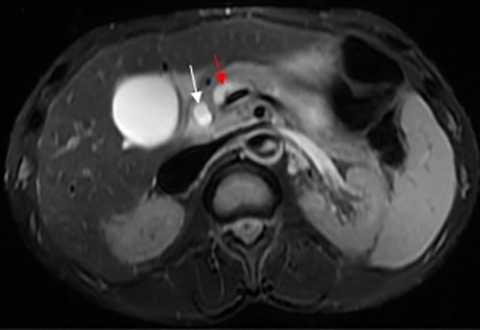
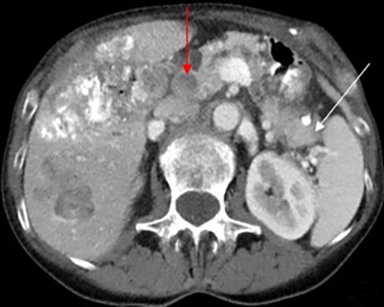
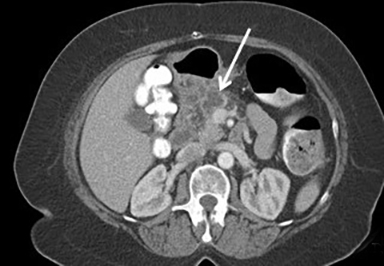
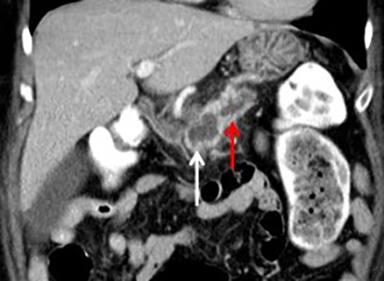
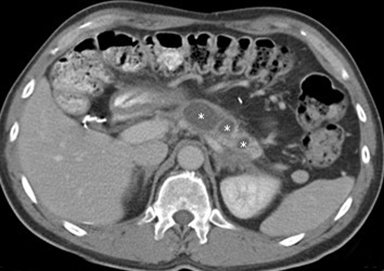
When a pancreatic mass is suspected, dual-phase (arterial and venous) contrast-enhanced CT or multiphase enhanced MRI is performed. Adenocarcinoma is typically most conspicuous on arterial phase images where it usually appears hypoattenuating relative to the enhancing pancreatic parenchyma. The arterial phase images are also used to assess for encasement of peripancreatic arteries (defined as tumor contact involving more than 180 degrees of vessel circumference). Venous phase images are optimal to evaluate for liver metastases and encasement or thrombosis of peripancreatic veins. Tumor in the pancreatic head may cause dilation of both the common bile duct and main pancreatic duct, creating a “double duct sign” (Figure 1). Approximately 5-11% of tumors may be isoattenuating to the pancreas on CT, in which case indirect findings may be helpful such as mass effect or abnormal convex contour of the pancreas, interruption of the pancreatic duct, dilation of the common bile and pancreatic duct, and atrophic distal pancreatic parenchyma.2 In addition, approximately 8% of pancreatic adenocarcinomas have cystic features.3 On MRI, adenocarcinoma is typically described as demonstrating low signal intensity on T1- and T2-weighted images due to its fibrotic nature. However, the lesion can atypically be heterogenous or mildly hyperintense on T2-weighted imaging (Figure 1).
The 2015 National Comprehensive Cancer Network guidelines describe the current criteria used to assess for tumor resectability.4 Important features to include in the radiology report include the size and location of the mass, the presence of pancreatic or biliary ductal dilation, degree of arterial and venous contact, the presence of venous thrombus, variant vascular anatomy, tumor invasion of local structures, and the presence of metastatic disease.5
Neuroendocrine tumors
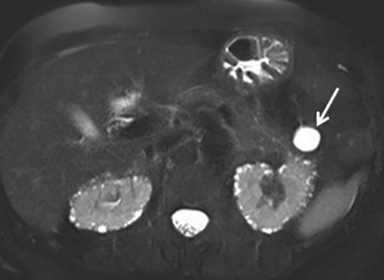
Pancreatic neuroendocrine tumors (NETs) represent approximately 1-5% of pancreatic neoplasms.1 Most occur sporadically, but approximately 1% to 2% are associated with familial syndromes.6 Pancreatic NETs are categorized as functioning or nonfunctioning tumors. Functioning tumors, which comprise approximately 15-52% of tumors, may produce symptoms related to excess hormone production. These include insulinoma, gastrinoma, VIPoma, somatostatinoma, and glucagonoma. Each of these tumors produces the hormone that is its namesake. The most common functioning NET is insulinoma. In general, functioning tumors tend to present earlier in the course of disease when they are small due to the clinical effects of excess hormone production, while nonfunctioning tumors present later due to mass effect. The risk of malignancy increases with tumor size. Approximately 90% of nonfunctioning tumors are malignant, while functioning tumors have variable malignancy rates.1
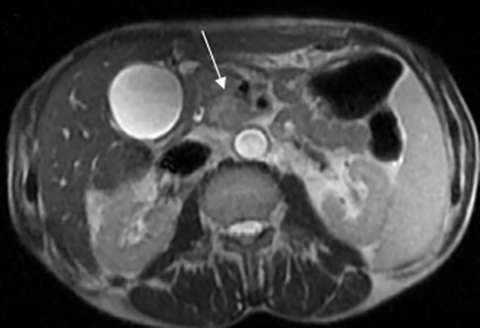
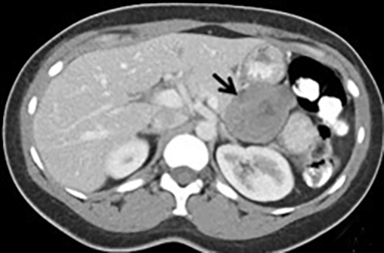
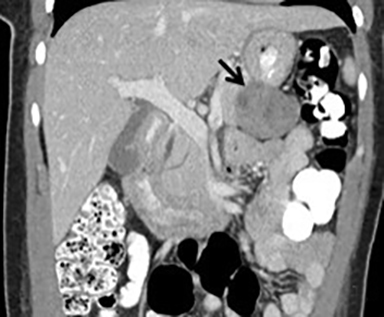
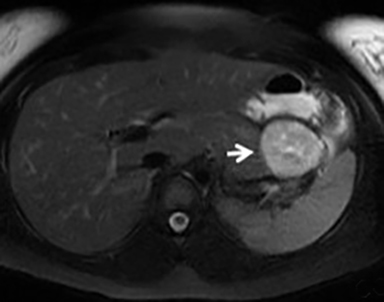
The imaging appearance of pancreatic NETs is variable. Smaller tumors tend to homogeneously enhance on arterial phase images and may be hyper-, iso-, or hypoenhancing relative to the pancreatic parenchyma on venous phase images. Larger lesions may demonstrate cystic degeneration and calcification, with calcification seen in 20% of NETs.1 Cystic NETs tend to have a peripheral hypervascular rim (Figure 2).7 Pancreatic ductal obstruction is uncommon. On MRI, most NET’s have low signal intensity on T1-weighted images and intermediate to high signal intensity on T2-weighted images.1 111 In-radiolabeled octreotide has high sensitivity for detecting NETs with the exception of insulinomas and poorly differentiated NETs.
Pancreatic Metastases
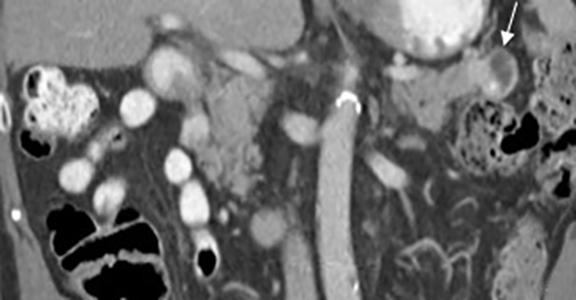
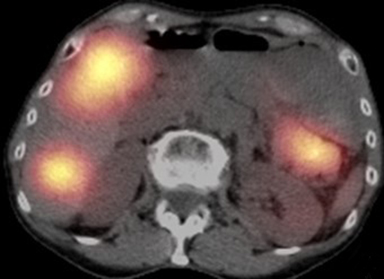
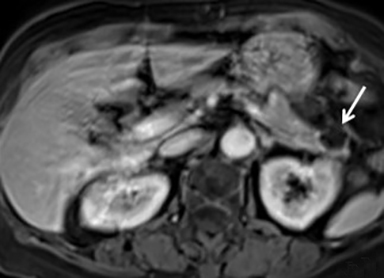
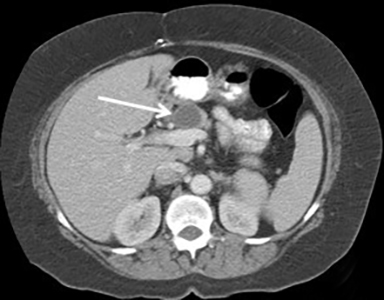
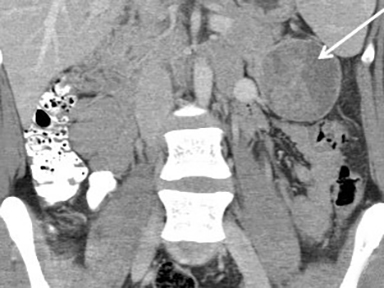
Pancreatic metastases account for approximately 2-5% of all pancreatic malignancies. Renal cell carcinoma and lung carcinoma are the most common primary tumors that metastasize to the pancreas, followed by breast and colorectal carcinomas and melanoma.1 Metastases are more commonly solitary rather than multiple and have imaging features related to that of the primary tumor (Figure 3).
Cystic masses
Serous cystadenoma
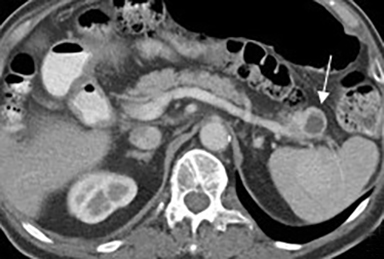
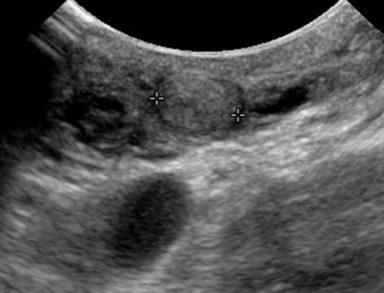
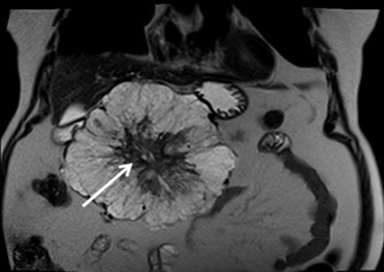
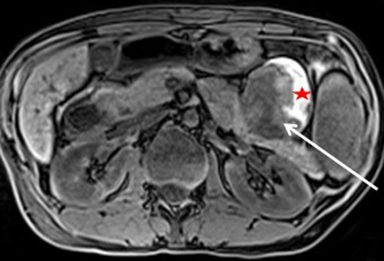
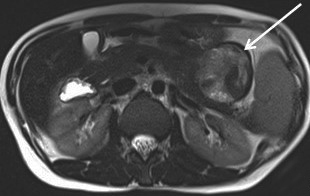
Serous cystadenomas are benign cystic neoplasms that comprise approximately 20% of pancreatic cystic lesions.8 Because approximately 75% occur in females with a mean age of 61, they are sometimes referred to as the “grandmother” lesion.9 Patients with von Hippel-Lindau disease may have multiple serous cystadenomas. However, this lesion can atypically occur in elderly males (Figure 4).
Serous cystadenomas typically have a microcystic appearance, consisting of multiple (>6) cystic locules, each less than 2 cm, lined by glycogen-rich epithelial cells and separated by fibrous septa. On CT, serous cystadenomas usually have a lobular border, do not typically cause pancreatic or biliary ductal dilation, and displace rather than invade adjacent structures.10 A calcified central scar is highly characteristic but only present in approximately 30% of patients (Figure 4).11 The presence of large numbers of microcysts may produce a solid appearance on CT.10 On MRI, the lesion appears as a cluster of small cysts, usually demonstrating simple fluid signal intensity, though occasionally showing T1-hyperintensity if there is hemorrhage. There is enhancement of the thin fibrous septa separating the cystic locules and the central scar. Calcification of the central scar may result in a signal void. There is no communication with the pancreatic duct.
The macrocystic or oligocystic variant of serous cystadenoma is uncommon and difficult to distinguish from mucinous cystic neoplasm by imaging.12 There is also an uncommon aggressive subtype, with 5.1% locally aggressive and 0.8% malignant in one series.13 Thus, imaging surveillance of serous cystadenomas is recommended with surgical referral if the lesion is symptomatic or larger than 4 cm.14
Mucinous cystic neoplasm (mucinous cystadenoma)
Mucinous cystic neoplasm (MCN) comprises 10% of pancreatic cystic neoplasms. It is sometimes referred to as the “mother lesion,” as approximately 95% occur in women with a mean age of 44-48.9 However, patients with mucinous cystadenocarcinoma are usually older, with a mean age of 55-64, possibly reflecting progression from adenoma to carcinoma.15,16
Most MCNs occur in the pancreatic body and tail. Pathologically, MCN is defined by the presence of ovarian-type stoma.15 MCN is a potentially malignant lesion, but it has a low incidence of carcinoma (12% invasive carcinoma and 5.5% in-situ carcinoma) with no malignancy seen in MCNs < 4 cm without mural nodules in one series.17
On CT and MRI, MCN typically appears as a well-circumscribed, smoothly marginated, unilocular or mildly septated macrocystic lesion (usually less than 6 locules, each larger than 2 cm) (Figure 5). Enhancing mural nodules, septal thickening, and septal and mural calcification are predictors of malignancy. Although MCN is mucin-filled, it usually demonstrates simple fluid signal intensity on MRI. MCN does not usually communicate with the pancreatic duct.18 MCN is typically a solitary lesion, so if additional cystic pancreatic lesions are present, intraductal papillary mucinous neoplasm or pseudocyst should be considered as an alternative diagnosis. Surgical resection is recommended in all surgically fit patients given the malignant potential of MCN and the relatively young age of most patients.19
Intraductal papillary mucinous neoplasm
Intraductal papillary mucinous neoplasms (IPMNs) comprise about 20% of pancreatic cystic lesions, are usually diagnosed in patients between 60 and 70 years old, and occur slightly more frequently in men.9,20 They are ductal epithelial neoplasms of mucin producing cells usually with papillary intraductal projections. Involved ducts are variably dilated and filled with mucin. IPMNs can show low-grade, intermediate-grade, or high-grade dysplasia or invasive carcinoma. Different grades of dysplasia can be seen within one lesion, which suggests the development of dysplasia from a lower to a higher grade.20
IPMNs are classified as side-branch IPMN, main-duct IPMN, or mixed IPMN involving both the main and side branches. Main duct IPMNs are more commonly malignant, with approximately 43% containing invasive carcinoma, while approximately 18% of side-branch IPMNs contain invasive carcinoma.19 Approximately 20-40% of IPMNs are multifocal.20
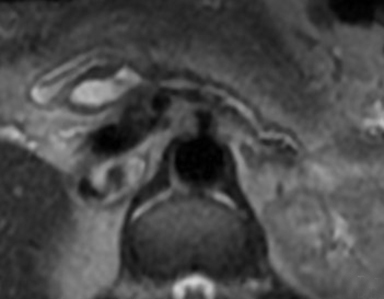
CT and MR evaluation of a main-duct IPMN shows diffuse or segmental dilation of the main pancreatic duct (Figure 6). Mural nodules, foci of intraductal mucin, or a dilated major or minor papilla protruding into the duodenal lumen may be seen. Pancreatic parenchymal atrophy may also be present. Side-branch IPMNs may be unilocular or multilocular with a macrocystic or microcystic appearance. Communication with the pancreatic duct is often demonstrated, particularly on MRI, and is a key imaging feature.21
Management of side-branch IPMNs is based on the presence of high risk stigmata (obstructive jaundice, enhancing solid component, or main pancreatic duct at least 10 mm in size) or worrisome features (pancreatitis, cyst at least 3 cm in size, thickened/enhancing cyst walls, main duct size 5-9 mm, nonenhancing mural nodule, or abrupt change in pancreatic duct caliber with distal pancreatic atrophy). Because of the higher incidence of invasive cancer in main-duct IPMNs, surgical resection is recommended for all surgically fit patients.19
Solid pseudopapillary tumor
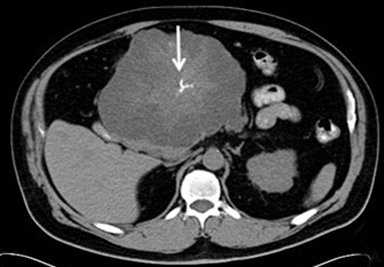
Solid pseudopapillary tumor (SPT) represents approximately 5% of pancreatic cystic lesions and almost exclusively occurs in young females, with a male to female ratio of 1:20 and a mean age of 25 years old 9; for this reason, it is sometimes referred to as the “daughter lesion.” SPTs have low malignant potential with more than 80% cured by surgical resection.9 SPTs typically are large, slow-growing masses, which show internal hemorrhagic and cystic degeneration. The classic CT appearance is a large well-encapsulated mass with solid and cystic components (Figure 7).22 Peripheral calcification is present in approximately 30% of cases. SPT’s are typically well-defined on MRI with variable signal intensity. Areas of increased T1 signal intensity are commonly seen due to hemorrhagic degeneration, and a fluid-fluid level is detected in 10-18% of cases related to the hematocrit effect. There may be a thick, low signal pseudocapsule on T1- and T2-weighted images. Enhanced images show early peripheral heterogeneous enhancement and progressive nonuniform enhancement on more delayed images.23
Pseudocyst
Pancreatic pseudocyst is the most common pancreatic cystic lesion. Pseudocysts are encapsulated by fibrous tissue without epithelium and usually form after inflammation, necrosis, or hemorrhage related to pancreatitis or trauma. The imaging appearance may vary based upon the age or contents of the pseudocyst. On CT, pseudocysts are usually unilocular round or oval fluid collections surrounded by a wall of variable thickness (Figure 8). Importantly, although the wall may enhance, there are no internal enhancing solid components. On MRI, pseudocysts may demonstrate simple fluid signal or increased signal on T1-weighted images if there are hemorrhagic or proteinaceous contents. There may be associated findings of acute or chronic pancreatitis, including peripancreatic inflammation, parenchymal calcifications or atrophy, and ductal dilation. Pseudocysts can often be treated conservatively, though drainage may be required in the setting of gastric outlet obstruction, biliary ductal obstruction, or infection.21
Revised Atlanta Classification of pancreatic fluid classifications has been recently published and defines a pancreatic pseudocyst as a fluid collection persisting greater than four weeks after a diagnosis of interstitial edematous pancreatitis. A fluid collection persisting greater than four weeks after necrotizing pancreatitis is recognized as walled off necrosis in the revised classification.
Mimics: Multiple lesions can mimic primary pancreatic masses, such as duodenal diverticula, accessory splenules in the pancreatic tail, retroperitoneal masses, or masses arising exophytically from adjacent organs (Figure 9).
Conclusion
Multiple solid and cystic pancreatic lesions are encountered on cross-sectional imaging. Knowledge of the typical imaging features, relevant clinical history, and patient populations affected is imperative to formulate an accurate diagnosis. Familiarity with CT and MRI appearance of pancreatic lesions is essential as entities listed in the differential diagnoses can change the clinical approach and prognosis. A multimodality approach is frequently needed to narrow the differential diagnosis, though tissue sampling is usually required for a definitive diagnosis.
Acknowledgement: The authors would like to acknowledge their use of MONTAGE, a radiology data mining and analytics software (Montage Healthcare Solutions Inc, Philadelphia, PA), to acquire some cases used in this publication.
References
- Ros PR, Mortelé KJ. Imaging features of pancreatic neoplasms. JBR-BTR. 2001;84(6):239–249.
- Kim JH, Park SH, Yu ES, et al. Visually isoattenuating pancreatic adenocarcinoma at dynamic-enhanced CT: frequency, clinical and pathologic characteristics, and diagnosis at imaging examinations. Radiology. 2010;257(1):87-96. doi: 10.1148/radiol.10100015. Epub 2010 Aug 9.
- Kosmahl M, Pauser U, Anlauf M, et al. Pancreatic ductal adenocarcinomas with cystic features: neither rare nor uniform. Mod Pathol. 2005;18(9):1157-1164.
- National Comprehensive Cancer Network http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed February 3, 2016.
- Al-Hawary MM, Francis IR, Chari ST, et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the society of abdominal radiology and the American pancreatic association. Gastroenterology. 2014; 146(1):291-304.e1. doi: 10.1053/j.gastro.2013.11.004.
- Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics. 2010;30(6):1445-1464. doi: 10.1148/rg.306105523. Review.
- Ligneau B, Lombard-Bohas C, Partensky C, et al. Cystic endocrine tumors of the pancreas: clinical, radiologic, and histopathologic features in 13 cases. Am J Surg Pathol. 2001;25(6):752-760.
- Ansari NA, Ramalho M, Semelka RC, et al. Role of magnetic resonance imaging in the detection and characterization of solid pancreatic nodules: An update. World J Radiol. 2015;28;7(11):361-374. doi: 10.4329/wjr.v7.i11.361. Review.
- Adsay NV. Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg. 2008;12(3):401-404. Epub 2007 Oct 24. Review.
- Khan A, Khosa F, Eisenberg RL. Cystic lesions of the pancreas. AJR Am J Roentgenol. 2011;196(6): W668-77. doi: 10.2214/AJR.10.4378. Review.
- Sarr MG, Kendrick ML, Nagorney DM, et al. Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am. 2001;81(3):497-509. Review.
- Chatelain D, Hammel P, O’Toole D, et al. Macrocystic form of serous pancreatic cystadenoma. Am J Gastroenterol. 2002;97(10):2566-2571.
- Khashab MA, Shin EJ, Amateau S, et al. Tumor size and location correlate with behavior of pancreatic serous cystic neoplasms. Am J Gastroenterol. 2011;106(8):1521-6. doi: 10.1038/ajg.2011.117. Epub 2011 Apr 5.
- Sahani DV, Kambadakone A, Macari M, et al. Diagnosis and management of cystic pancreatic lesions. AJR Am J Roentgenol. 2013;200(2):343-354. doi: 10.2214/AJR.12.8862. Review.
- Reddy RP, Smyrk TC, Zapiach M, et al. Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol. 2004;2(11):1026-1031.
- Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231(2):205-212.
- Crippa S, Salvia R, Warshaw AL, et al. Mucinous cystic neoplasm of the pancreas is not an aggressive entity: lessons from 163 resected patients. Ann Surg. 2008;247(4):571-579. doi: 10.1097/SLA.0b013e31811f4449.
- Le Borgne J, de Calan L, Partensky C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French Surgical Association. Ann Surg. 1999;230(2):152-161.
- Tanaka M, Fernández-del Castillo C, Adsay V, et al. International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183-197. doi: 10.1016/j.pan.2012.04.004. Epub 2012 Apr 16.
- Castellano-Megías VM, Andrés CI, López-Alonso G, et al. Pathological features and diagnosis of intraductal papillary mucinous neoplasm of the pancreas. World J Gastrointest Oncol. 2014;6(9):311-324. doi: 10.4251/wjgo.v6.i9.311. Review.
- Kucera JN, Kucera S, Perrin SD, et al. Cystic lesions of the pancreas: radiologic-endosonographic correlation. Radiographics. 2012 Nov-Dec;32(7): E283-301. doi: 10.1148/rg.327125019.
- Choi JY, Kim MJ, Kim JH, et al. Solid pseudopapillary tumor of the pancreas: typical and atypical manifestations. AJR Am J Roentgenol. 2006;187(2): W178-86.
- Low G, Panu A, Millo N, et al. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. Radiographics. 2011;31(4):993-1015. doi: 10.1148/rg.314105731.
Dr. Mehta and Dr. Dorff are Radiologists at the Hospital of the University of Pennsylvania, Philadelpha, PA. The authors have no grants, disclosures, or other assistance to declare.
