Applied hepatobiliary scintigraphy in chronic gallbladder diseases
Images



This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
Chronic gallbladder-related conditions often manifest in abdominal biliary-type pains – also called biliary colic. As with any symptom, biliary colic is subjective and challenging to distinguish from abdominal pain originating from other organs. Hence, multiple imaging tests are used in what often ends up as a protracted diagnostic pursuit. This article will address the role of hepatobiliary scintigraphy (HBS) in the diagnosis and management of this often challenging clinical conundrum.
The terminology and disease nomenclature applicable in this context could be confusing. The starting point is differentiation of chronic gallbladder (GB) diseases based on their main pathophysiologcal sine qua non, which can be either structural (anatomical) or functional. In the former group one can directly visualize the culprit abnormality or its sequelae on anatomical imaging, such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). The classic example of a structural condition would be chronic calculous cholecystitis (CCC) where US plays a central role by demonstrating stones in the gallbladder and other associated findings. However, gallstones seen on US could also be totally asymptomatic and demonstration of poor GB contractility could differentiate incidental gallstones from those associated with symptomatic CCC.1,2 In the functional group, there is typically no anatomical abnormality on imaging or even on the pathological specimen, such as in the case of functional gallbladder disorder (FGBD). This is where the diagnosis is based on characteristic symptoms and abnormal GB function, typically revealed by cholecystokinin HBS (CCK-HBS).3 Other chronic conditions could be responsible for abdominal pain that is often clinically indistinguishable from the biliary colic. Some of them can be discerned from careful examination of CCK-HBS for non-GB clues.
Radiopharmaceuticals, pharmaceuticals and physiology
Development of Tc-99m labeled iminodiacetic acid (IDA) derivatives4 provided us with an elegant way to study major elements of hepatobiliary physiology, pathophysiology and response to various stimuli. We can depict liver blood flow, hepatocellular function, bile formation and excretion into the bowel, as well as biliary tract dynamics of its bowel transit.
Hepatobiliary radiopharmaceuticals underwent remarkable evolution that is detailed elsewhere.5,6 Perfection of IDA derivatives led to development of the modern analogues that are probably the closest we have come in nuclear medicine to the concept of an ideal radiopharmaceutical.7 They are actively taken up and transported intracellularly by hepatocytes’ organic anion-transporting polypeptide (similar to non-conjugated bilirubin). They are later excreted into canaliculi unchanged via apical ATP-dependent export pump. Tc-99m Disofenin or 2,6-diisopropylacetanilido iminodiacetic acid (DISIDA) and Tc-99m Mebrofenin or bromo-2,4,6-trimethylacetanilido iminodiacetic acid (BromIDA) are the two most commonly used today. Considering a patient’s total bilirubin level, a clinical question addressed by the test, availability and the cost, one can make the specific choice. If the latter two are of no concern, Tc-99m Mebrofenin is the ideal choice, for it has the best hepatic uptake and washout, while displaying the least activity outside the hepatobiliary system (vicarious excretion).5
A typical adult dose is 200 MBq (5 mCi) of either compound injected as an intravenous bolus. BromIDA should be used in jaundiced patients, escalating the dose to 7.5 mCi and 10 mCi if the total bilirubin level reaches 4 mg/dl and 8 mg/dl, respectively. Pediatric patients should receive 7 MBq/kg (0.2 mCi/kg), but no less than 37 MBq (1 mCi). The gallbladder wall is the target radiation exposure organ, receiving 0.11 mGy/MBq dose.8
The existence of cholecystokinin (CCK), a gastrointestinal hormone, was first suggested by physiologist Joy Simcha Cohen in 1905. Since then it became clear that CCK represents a family of peptides that vary in number of amino acids. It is the C-terminus with sulfate-group attached to the tyrosine in position 7 that is common to all of the family members and responsible for their hepatobiliary actions upon binding to CCK-A receptor. CCK is synthesized and released by the mucosal lining cells of the duodenum and jejunum. The stimulus for its release is the entry of partially digested fats and proteins into the duodenum. CCK then hematogenously reaches the receptors of the liver (increasing blood flow and production of bile), pancreas (increasing production of pancreatic juice), the gallbladder (contracting its smooth muscle), and the sphincter of Odd (relaxing it to allow bile and pancreatic juice to flow into the duodenum). When bile and pancreatic juice digest fats and proteins in the small intestine, the stimulation for release of CCK ends. Sincalide is a synthetic C-terminal octapeptide analogue of CCK that is readily available commercially for parenteral use, which is used almost exclusively for diagnostic use in CCK-HBS.
Technique of hepatobiliary scintigraphy
Patient preparation
Ensuring an optimal scintigraphic result starts with patient preparation. The biliary flow and GB motility is a complex process that can be dramatically altered by ingested food and medications. The patient must fast for at least four hours prior to the test. As explained above, most meals would contract the GB that could last a long time and radioactive bile would not be able to enter against the outgoing flow and high pressure generated by the contracted GB, possibly resulting in non-visualization (non-viz) of GB. The last meal before a routinely scheduled morning test should occur 9-10 PM and should contain a significant fatty and/or protein component that the patient should be able to tolerate. This should empty the GB overnight, rendering it in the state of refilling at the start of scintigraphy in the morning. This ensures a prompt and optimal filling of GB with radioactive bile during the test. Fasting for 12 hours or longer, on the other hand, the patient will have a partially filled GB and tracer appearance may be delayed and/or suboptimal. By 24 hours of fasting, the GB would be filled to capacity and may not accept anymore secreted bile, causing GB non-viz during the test. This can be avoided by pretreating such patients with sincalide, infusing the total dose of 0.02 μg per kilogram over 15 to 60 minutes intravenously. The longer the infusion duration, the greater the emptying.9 Greater emptying is expected to prompt greater refilling with radioactive bile during the test. Because sincalide has a short duration of action, the HBS can start 15-20 min after sincalide infusion is completed. This preparation maneuver does not change subsequent sincalide stimulated GBEF.10
Patients’ medications must be screened for interaction and/or interference with CCK-HBS. The most impactful are opiates and opioid drugs, which must be discontinued for at least 4 half-lives of a given medication. They interfere with GB contractility by constricting the sphincter of Oddi (SO), which in turn increases resistance to GB emptying, preventing its effective contraction. They may also interfere directly with GB smooth muscle contractility. Other medications that interfere with GB contraction and should be withheld, if possible, include anticholinergic drugs, calcium channel blockers, oral contraceptive agents, histamine-2 receptor antagonists, and benzodiazepines.11
Imaging specifications
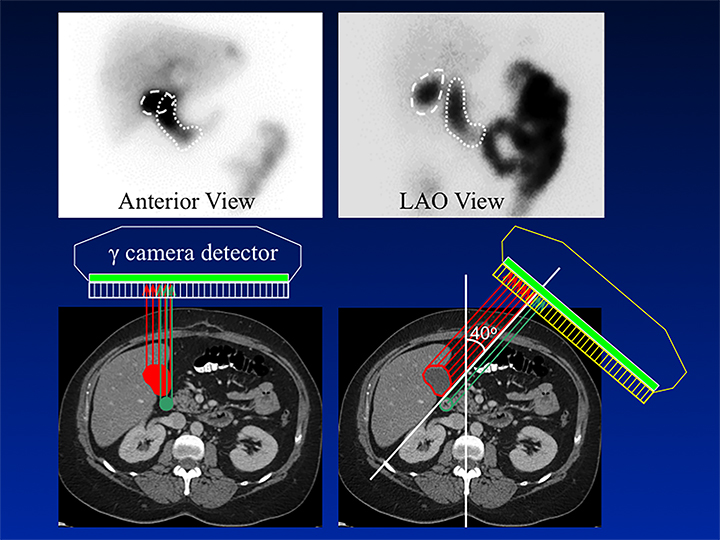
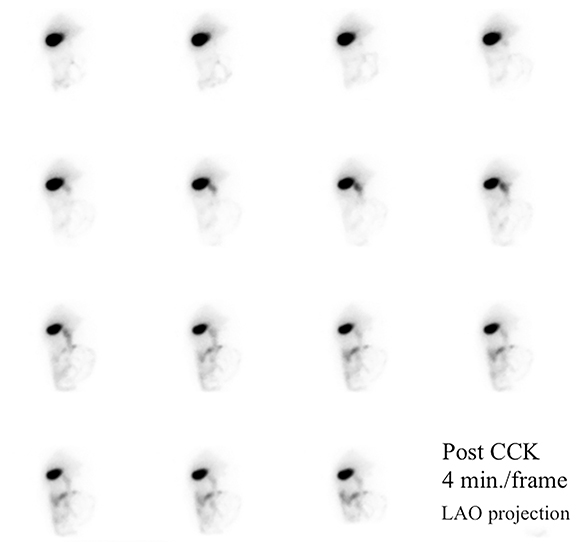
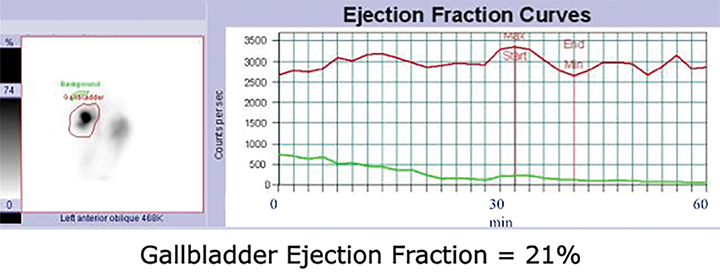
HBS includes an optional rapid blood flow (scintiangiography) phase and a slower dynamic (hepatobiliary) baseline phase. Optimal resolution and counting statistics can be obtained by acquiring images in a 128 x 128 matrix matrix. The framing rate for the scintiangiography is one frame per second for 60 seconds, while the subsequent images for the parenchymal phase are acquired at one frame per 15 seconds for one hour. The flow is best viewed by re-framing the rapid phase into 3-5 seconds per displayed frame, while the slower dynamic parenchymal and biliary phases are re-framed into 2-4 minutes per displayed frame. If further dynamic imaging is required following intervention, such as for imaging during sincalide stimulation of the GB (stimulation phase), it is typically acquired and displayed similar to the hepatobiliary baseline phase. To minimize duodenal activity interference with GB activity measurements for ejection fraction (GBEF) calculation, the images are usually acquired in 35 - 40° left anterior oblique projection, which renders the best separation of GB from duodenal activity (see Figure 1). Individualization on the basis of initial imaging may be needed in those who may have unusual GB position (intrahepatic, vertical-posterior, etc.). SPECT, and particularly SPECT/CT, could sometimes be useful in resolving equivocal findings on delayed phases of HBS; especially in rare cases of ascertaining that visualized activity accumulation is located within atypically positioned GB.12
Qualitative assessment of hepatobiliary scintigraphy
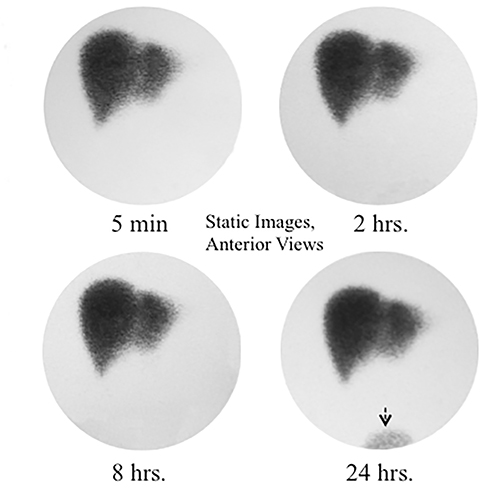
The scintiangiography phase may reveal gross abnormalities of the heart and the aorta, such as cardiomegaly or aneurisms.13 Liver blood flow via the hepatic artery is typically faint, as it represents only 25% of overall blood circulation through the organ. Activity in the liver begins to accumulate more rapidly upon recirculation, as blood returns via the portal vein 3 to 5 seconds later. It is just before the portal phase that a focal blush signals a lesion with arterial hypervascularization, such as hepatocellular carcinoma, adenoma, or focal nodular hyperplasia. Conversely, decreased flow can be seen in hypovascular lesions exemplified by a cyst.14
Next is the hepatocellular or parenchymal imaging phase. The first 8-10 minutes of imaging offers a window into the functional hepatocellular integrity. Normally, the blood pool activity in the heart clears completely by the 8th minute (it may be faintly seen on the first 4-minute image), with the tracer concentrated densely in the liver and complete disappearance of cardiac activity. In cases of severe hepatocellular disease (hepatitis, cirrhosis, etc.) the cardiac blood pool activity can persist for hours following the injection. Avid tracer uptake allows one to examine the liver for a hepatocyte-replacing, space-occupying lesion (such as metastatic disease, hemangioma, liver abscess, liver cyst, etc.). An appearance of the tracer in the ductal system signals the beginning of the biliary dynamic phase. While the left and right hepatic ducts are typically seen, visualization of the segmental hepatic ducts is rare in the normal individual and may indicate pathology. Biliary tree dilation can range anywhere from a slight residual prominence in a previously obstructed system to a more pronounced appearance in a partial obstruction, such as sphincter of Oddi stricture or dysfunction.
When the bile enters the duodenum it signals the beginning of the intestinal phase. The time from the radiotracer injection to this phase is commonly called a “biliary-to-bowel transit time.” Depending on the bile production rate and driving pressure gradients in the hepatobiliary system, this time may be as short as 10-15 minutes or as long as 1-2 hours. A recent meal, stimulating a lasting GB contraction with increasing bile flow, represents an example of the former.15 In counter distinction, CCK administration 20 min before the study or stimulating meal given 4-10 hours before the study usually causes preferential bile flow into the relaxing GB with some activity in CBD to the point of physiologically still constricted SO, which does not allow bile into the duodenum.16 In some such cases activity in the duodenum may not be seen for up to 2 hours. A common response this observation in practice is to wait for activity in the intestine before giving sincalide for CCK-HBS, supposedly to avoid stimulating GB in cases of pathological CBD obstruction. The imaging characteristics of physiological and pathological delay of duodenal activity are distinctly different, as shown in Figure 2. There is no reason to delay CCK administration by waiting for activity appearance in the duodenum or small bowel if the imaging demonstrates pattern of physiological delay. It is safe to proceed to CCK stimulation, particularly if the patient has no acute symptoms. Finally, the bile production rate plays an important role, explaining severe transit time delays in cases of nonobstructive intrahepatic cholestasis or severe hepatocellular disease.
As the tracer fills the biliary system and the bowel, a concomitant parenchymal washout occurs that can be expressed numerically by T1/2. The washout is normally homogeneous throughout the liver. However, abnormally slow washout can be seen diffusely or focally. The former is best exemplified by hepatocellular dysfunction with intrahepatic cholestasis. The latter is typically seen in nodular hyperplasia (FNH), but also in hepatic adenoma and, rarely, in hepatocellular carcinoma. These three entities cannot be definitively differentiated by scintigraphy, but some general rules do apply. A typical triad for FNH consists of increased flow on scintiangiography phase (76% of all FNH cases), increased or normal uptake of Tc-99m Sulfur Colloid and IDA compound, with frequent (92%) delayed focal washout on HBS.17,18Adenoma typically has unremarkable flow and reduced Tc-99m Sulfur Colloid uptake. Hepatocellular carcinoma usually has increased arterial flow, reduced Tc-99m Sulfur Colloid and IDA uptake, with rare instances of very slow washout that shows up as a “hot spot” on delayed HBS when normal parenchymal activity clears. However, the clinical value of these observations is limited, as there are no reliable data on sensitivity and specificity for these patterns.
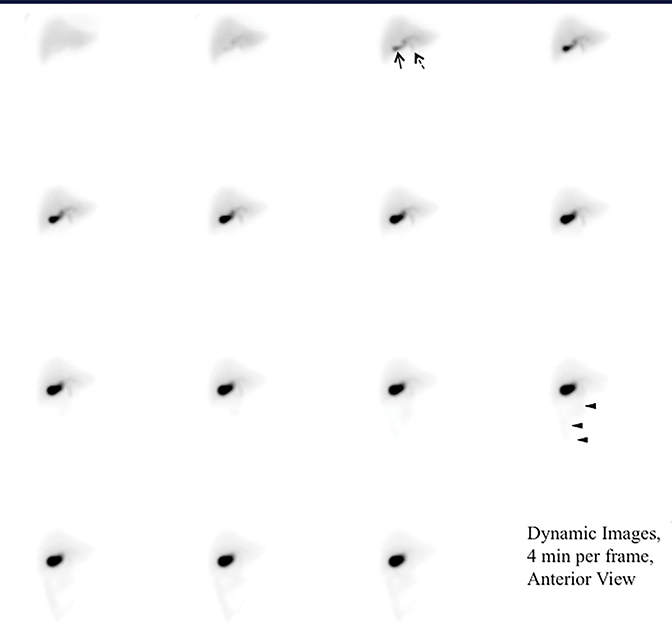
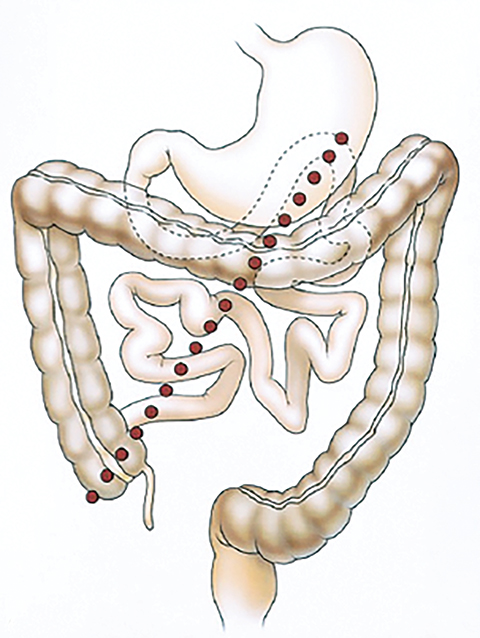
Appearance of activity in the duodenum heralds the beginning of the intestinal phase on HBS. It is important to assess the pattern of bowel activity on all images. The most clinically consequential abnormal pattern of small bowel activity is visualizing activity in the second part of the duodenum on the patient’s right side with failure to cross the midline, which strongly suggests the diagnosis of malrotation, as demonstrated in Figure 3. Such patients may present with abdominal pain that is clinically indistinguishable from the biliary colic. Symptomatic intestinal malrotation is a surgically curable cause of abdominal pain and can be identified on HBS, if a reader makes checking for duodenal activity going across the midline a habit.
Enterogastric reflux is a common incidental finding on HBS, depicting bile activity in the stomach. One study suggested that it is associated with chronic gastritis, which would be important to mention as it may explain abdominal symptoms in some patients.19 Much less common is a finding of photopenic defects in the GB, which is most likely due to larger gallstones20 and rarely could be mimicked by a bowel loop compressing a GB.21 Another rare finding is a large abdominal mass that appear as a persistent focus of photopenia in the midst of activity-filled bowel loops.22 An unusually dilated small bowel loop that fills only to a point may signal the intestinal obstruction,23 and a markedly dilated stomach filled with refluxed radioactive bile could be a secondary finding of a small bowel obstruction.24
Quantitative Analysis in Hepatobiliary Scintigraphy
By far the most common analytical application to HBS is quantitation of GBEF. It is the difference in GB counts, corrected by the background activity, between its maximal and minimal intensity, as a percent of the former. While it is a simple calculation, care must be taken to confirm that the GB region of interest (ROI) contains the GB throughout this imaging segment. First, the images should be obtained in projection that has least likelihood of GB overlap with other structures containing activity of which duodenum is the most important. Whenever available, examination of axial tomographic images, such as CT or MRI, should provide sufficient guidance in selecting projection. In the typical anatomic arrangement of the abdominal organs the best projection for such imaging is around 35 to 40 degree left anterior oblique view (Figure 1).
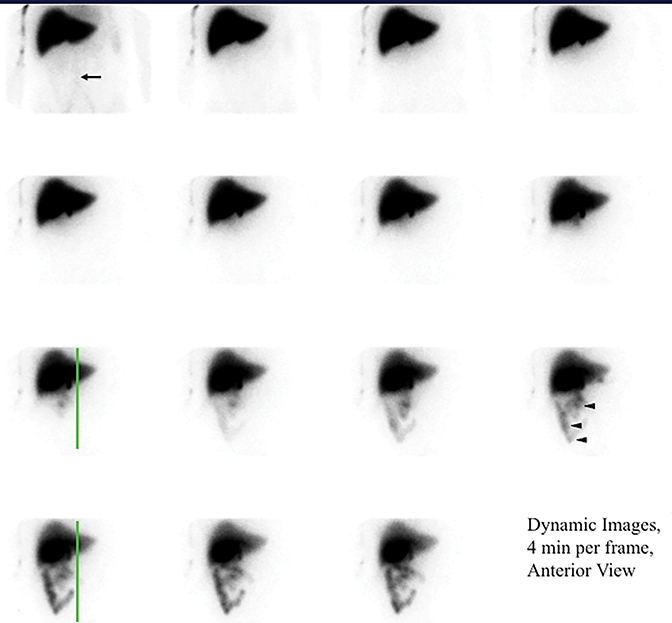
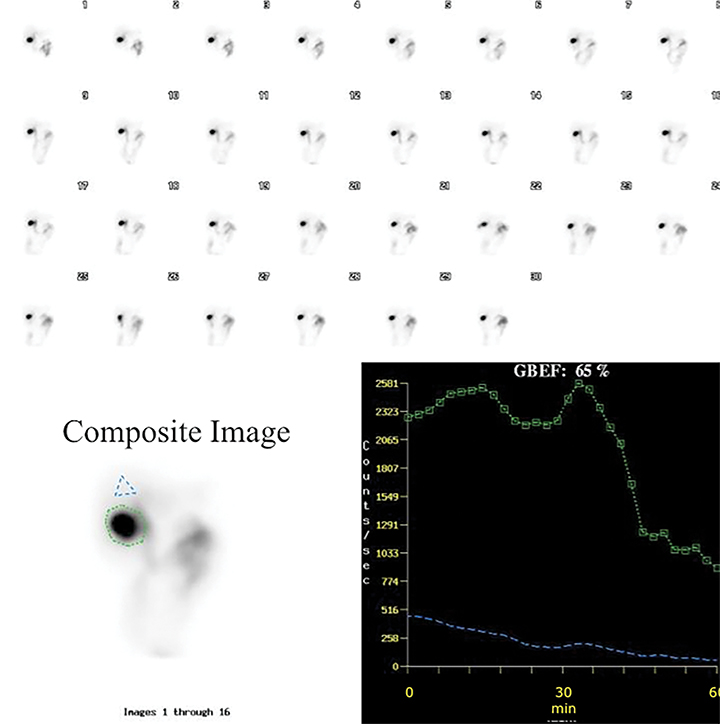
It is common for the GB to change orientation with CCK infusion without any movement on the part of the patient, usually with the GB fundus moving up in the cranial direction and the GB assuming a more horizontal position. Such motion may cause a partial escape of GB outside the ROI, which is commonly drawn on the early image and applied to the entire image set, leading to an erroneously higher GBEF. Patient motion can have a similar effect by moving the GB outside a stationary ROI, as shown in Figure 4. It is important for quality assurance to apply individual GB and background ROIs to each representative frame of post CCK phase of HBS to avoid making such a mistake. Inappropriately positioned background ROI that includes bowel activity could also occasionally lead to an erroneous result. Another common problem with stationary GB ROI is an unintentional inclusion of nearby bowel activity that moves into the ROI towards the end of the study. All of these problematic and misleading results can be avoided by visually inspecting post-CCK images with ROIs displayed. This must be available on all contemporary gamma camera systems along with quantitative GBEF application. Interestingly, some colleagues advocate only visual (qualitative) assessment of images for characterization of gallbladder emptying.25 Visual inspection is an important component of evaluation, but in contemporary nuclear medicine practice it is difficult to find justification for omitting computer processing for GBEF in favor of visual inspection alone.
It is critical to standardize administration of sincalide to accurately assess GBEF. The SNMMI and inter-specialty groups have endorsed administration of 0.02 micrograms of sincalide over 60 minutes.11,26 The normal GBEF with the above recommended infusion rate is 38% or greater. This provides the least variability and the highest specificity as compared to shorter infusion times.9 Taking a historical GBEF cutoff of 35% as normal, there are 27% and 10% false positive rates when the total dose of sincalide is infused over 15 and 30 min in normal volunteers, respectively.9 The other finding of the study on infusion methodology conducted in 60 normal subjects is that all of those subjects showed GB activity by 60 min after radiotracer administration, and they all did so on three different occasions.9 By inference, this should tell us that if GB is not visualized by 60 min in a well-prepared patient, it is evidence for abnormally functioning GB. Therefore, it is recommended to not pursue those cases with delayed imaging or especially the administration of morphine sulfate in order to visualize activity in the GB. It serves no purpose, except maybe in a rare instance of incidentally occurring acute cholecystitis in a patient referred for chronic symptoms for the GBEF study. Such patient should be easily identifiable by a basic bedside evaluation.
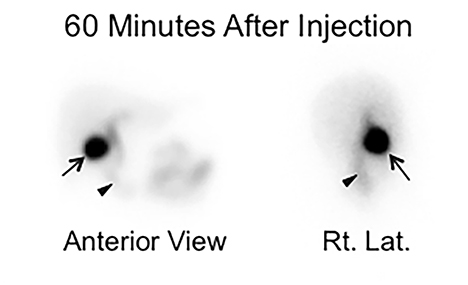
It is common to image dynamically for the first hour after injection of the radiotracer. However, the first-hour imaging is of limited additive information when the goal is to determine GB function, and some have substituted one or few static views to confirm activity in the GB prior to injecting CCK.27 We recently presented retrospective blinded reviews of our clinical experience, demonstrating that substituting the first-hour dynamic anterior view imaging with 2 static views (anterior and right lateral, Figure 4) obtained one hour after radiotracer injection that resulted in no significant diagnostic loss or misses.28
Clinical applications
In principle, the GBEF is used to identify abnormally functioning GB (decreased GBEF; ie, GB hypokinesia) irrespective of the pathology causing the chronic symptoms. This group of conditions is rather heterogeneous and the key symptom is a chronic, periodic abdominal pain, often biliary-like in character (“colicky”). First described with the help of cholecystokinin cholecystography,29-32 it remains a highly debated and evolving entity.33
It is attractive to consider GBEF as the objective differentiator between those who may respond to cholecystectomy from those who are not likely to be helped by this surgery. Most studies done to investigate this clinical application do not sub-select the subjects on the basis of pathology or lack thereof, but instead take all comers with abdominal symptoms that could have been biliary in origin. The other limitation is that all investigations on this topic, except for one study of patients with acalculous biliary pain,34 are retrospective. There are multiple factors, described below, that can influence GB contractile function and need to be considered as possible causes of false positive or false negative results, which would be difficult to identify in retrospective studies.
Application of GBEF in hospitalized patients who are typically acute, undergoing active medical treatment, and often suffering from nausea, abdominal pain and other gastrointestinal symptoms warrants a word of caution. It is likely that in such cases GBEF may be abnormal as a result of pharmacological, hormonal, or neural influences, causing significant reduction in specificity of the test.35,36 Therefore, experts recommend using this test in clinically stable outpatients only37 For example, increased GB contractility is observed with cholinergic agonists,38,39 hypercalcemia40 erythromycin41 nonsteroidal anti-inflammatory drugs42 and those with vagotomy.43 These circumstances may promote false-negative results. But false-positive studies are probably even more common and can result from reduced GB contractility secondary to opioids44 endotoxins associated with severe intercurrent illness45 hyperglycemia,6 somatostatin47-51 diabetic neuropathy,52 spinal cord injury,53 achalasia54 inflammatory bowel syndrome,55 liver cirrhosis56 and progesterone therapy57
It is because of this often inconspicuous complexity that one finds such a diversity of results in the overwhelmingly retrospective literature. However, the majority of studies report high value of abnormal GBEF in predicting success of cholecystectomy for the pain relief,34,58-66 while a minority offer opposing views.1,67,68
Functional gallbladder disorder (FGBD)
The current principal criteria for the diagnosis of FGBD includes biliary pain and absence of GB stones or other structural pathology.3 The low GBEF was included as the supportive criteria for the diagnosis of FGBD in 2016.3 The other supportive criteria includes normal liver enzymes, normal conjugated bilirubin, and normal amylase/lipase.3 This disorder is of yet unknown etiology. It is important to recognize that designation of FGBD a disorder emphasizes abnormality or disturbance of function. There is a great deal of confusion in the literature that contains numerous names for it that were developed over the years and by different specialties. These include “biliary dyskinesia,” “GB dyskinesia,” “chronic acalculous cholecystitis,” “acalculous cholecystopathy,” “chronic acalculous biliary disease,” “acalculous biliary disease,” and probably some others. It is therefore not unusual to find one of the above names in reports of CCK-HBS. The multispecialty consensus statement of 2011 offered the following recommendation for the CCK-HBS impression statement in cases with abnormal GBEF and normal anatomical imaging findings: “Abnormal GBEF of X% is consistent with functional gallbladder disorder in the proper clinical setting.”11 It is important to adhere to this recommendation in order to improve consistency of our reporting.
Chronic calculous cholecystitis
Presence of gallstones (cholelithiasis) in the general population is as high as 1 in every 5 people.69 They are classified into asymptomatic and symptomatic. Establishing asymptomatic cholelithiasis is obvious when there are no abdominal complaints. But the seemingly simple division is complicated in many patients with abdominal pain because of inherent challenges in eliciting and interpreting subjective symptoms. The combination of typical chronic biliary symptoms and anatomical demonstration of cholelithiasis is a reliable evidence of chronic cholecystitis, requiring no further diagnostic evaluation prior to cholecystectomy. However, additional diagnostic testing may be useful in patients with atypical abdominal symptoms and cholelithiasis in order to affirm causal relationship by demonstrating abnormal GBEF. Administration of sincalide in patients with cholelithiasis could be viewed by some professionals as unsafe for the concern of dislodging a stone and precipitating biliary tract obstruction and/or pain. The fact remains that there are studies that used sincalide in patients with known gallstones and none reported obstructive complications. Abdominal pain was reported in 1/67 patients with gallstones during sincalide infusion.1 The consensus of specialists also found no evidence for this concern and considered sincalide testing safe.11 The literature experience shows that majority of patients (>75%) with gallstones and abdominal symptoms have normal GBEF.2 This means that their abdominal pain is of non-GB etiology. On the other hand, abnormal GBEF was a strong predictor of biliary pain recurrences.
Non-gallbladder findings leading to cause of abdominal pain
It is important to carefully examine the images for other potential causal findings. The finding of the malrotation, as shown in Figure 3, is one such causal finding that while very rare is definitely most consequential. Another finding to watch out for is increased peristalsis that may indicate irritable bowel syndrome when activity transits rapidly into the colon after sincalide administration.70 This finding needs to be clinically correlated by the referring service. In many cases one can observe some duodenogastric reflux of bile, which when prominent should be suggested as a potential cause of bilious gastritis.71
Practical interpretation algorithm
In patients with chronic abdominal pain and abnormal GBEF on CCK-HBS, the pertinent information should be queried for the absence or presence of gallstones and/or sludge. If an anatomical study is normal, the most appropriate interpretation would be to suggest the diagnosis of FGBD. If, on the other hand, there is presence of stones and/or sludge, the interpretation should implicate chronic calculous cholecystitis. While this represents a simple algorithm, it is probably too simple to fully capture the clinical reality. It is well understood that with time the poor motility of the GB observed in FGBD may lead to formation of sludge and later probably results in GB stones. Yet the above interpretational algorithm offers a reasonable and a logical approach. In those with normal CCK-HIDA, it is important to scrutinize the study for causal non-GB findings.
Conclusion
HBS continues to enjoy frequent application in clinical gastroenterology, particularly in the workup of chronic biliary pain. The most appropriate indication remains suspected FGBD in patients with biliary-type or atypical chronic abdominal pains and negative findings on anatomical imaging. The preponderance of evidence is in favor of using abnormal GBEF as a pathophysiological rationale for identifying abnormal GB function in these patients and using this finding for selecting patients for cholecystectomy. Adherence to the standard GB stimulation methodology is critical for preventing false-positive results and calls for administration of 0.02 micrograms of sincalide per kilogram of body weight that should be infused over 60 minutes. However, more evidence is needed to establish utility of this test in patients with cholelithiasis in the era of optimized sincalide infusion standardization.
references
- Gani JS. Can sincalide cholescintigraphy fulfil the role of a gall-bladder stress test for patients with gall-bladder stones? Aust N Z J Surg. 1998; 68(7):514-519.
- Hong SN, Lee JK, Lee KT, et al. Usefulness of gallbladder ejection fraction estimation to predict the recurrence of biliary pain in patients with symptomatic gallstones who did not undergo cholecystectomy. Dig Dis Sci. 2004;49(5):820-827.
- Cotton PB, Elta GH, Carter CR, Pasricha PJ, Corazziari ES. Rome IV. Gallbladder and Sphincter of Oddi Disorders. Gastroenterology. 2016.
- Loberg MD, Cooper M, Harvey E, Callery P, Faith W. Development of new radiopharmaceuticals based on N-substitution of iminodiacetic acid. J Nucl Med. 1976;17(7):633-638.
- Krishnamurthy GT, Turner FE. Pharmacokinetics and clinical application of technetium 99m-labeled hepatobiliary agents. Semin Nucl Med. 1990;20(2):130-149.
- Hladic III WB, Norenberg JP. Radiopharmaceuticals for Hepatobiliary Imaging. In: Robert E. Henkin MD, F.A.C.N.P., ed. Nuclear Medicine. Vol II. First ed: Mosby; 1996:986-996.
- Karesh SM. Principles of Radiopharmacy. In: Robert E. Henkin MD, F.A.C.N.P., ed. Nuclear Medicine. Vol I. First ed: Mosby; 1996:334-349.
- Gilbert SA, Brown PH, Krishnamurthy GT. Quantitative Nuclear Hepatology. J Nucl Med Technol. 1987;15(1):38-43.
- Ziessman HA, Tulchinsky M, Lavely WC, et al. Sincalide-stimulated cholescintigraphy: a multicenter investigation to determine optimal infusion methodology and gallbladder ejection fraction normal values. J Nucl Med. 2010;51(2):277-281.
- Sostre S, Canto MI, Kalloo AN. Gallbladder response to a second dose of cholecystokinin during the same imaging study. Eur J Nucl Med. 1992;19(11):964-965.
- Dibaise JK, Richmond BK, Ziessman HH, et al. Cholecystokinin-Cholescintigraphy in Adults: Consensus Recommendations of an Interdisciplinary Panel. Clin Gastroenterol Hepatol. 2011;9(5):376-384.
- Sood R, Murguia J, Graham MM, et al. A diagnostic dilemma of atypical gallbladder appearance on Tc-99m HIDA cholescintigraphy resolved with SPECT/CT. Clin Nucl Med. 2011;36(2):160-163.
- Stryker J, Siegel A. Abdominal aortic aneurysm visualized with hepatobiliary scintigraphy. Clin Nucl Med. 1997;22(9):645-646.
- Yeh SH, Shih WJ, Liang JC. Intravenous radionuclide hepatography in the differential diagnosis of intrahepatic mass lesions. J Nucl Med. 1973;14(8):565-567.
- Qvist N, Oster-Jorgensen E, Rasmussen L, Hovendal C, Pedersen SA. Postprandial gallbladder filling: relation to gastrointestinal motility. Scand J Gastroenterol. 1989;24(8):969-974.
- Kim CK, Palestro CJ, Solomon RW, Molinari DS, Lee SO, Goldsmith SJ. Delayed biliary-to-bowel transit in cholescintigraphy after cholecystokinin treatment. Radiology. 1990;176(2):553-556.
- Tanasescu D, Brachman M, Rigby J, Yadegar J, Ramanna L, Waxman A. Scintigraphic triad in focal nodular hyperplasia. Am J Gastroenterol. 1984;79(1):61-64.
- Boulahdour H, Cherqui D, Charlotte F, et al. The hot spot hepatobiliary scan in focal nodular hyperplasia. J Nucl Med. 1993;34(12):2105-2110.
- Wang GX, Shih WJ, Tang PL, Dian XH, Zhang WJ. Duodenogastric reflux demonstrated by cholescintigraphy in peptic ulcer disease and chronic gastritis. Clin Nucl Med. 1994;19(2):100-103.
- Shih WJ, Domstad PA, DeLand FH, Beihn R, Stipp V. Cholescintigraphy in the detection of cholelithiasis: a review of 23 cases and a case report demonstrating the sequential evolution of the appearance of the gallbladder defect. Eur J Nucl Med. 1986;12(5-6):299-301.
- Hoffman EJ, Tulchinsky M, Eggli DF. Bowel compression on the gallbladder mimicking a gallstone on cholescintigraphy. Clin Nucl Med. 1997;22:709-710.
- Lee MJ, Zuckier LS, Zalta B, Fine E. The persistently absent bowel loop: a sign of mass effect on the bowel detected by cholescintigraphy. Clin Nucl Med. 2001;26(11):939-940.
- Tobin M, Velchik MG, Powe J, Hibbard C, Alavi A. The cholescintigraphic pattern of small bowel obstruction. Clin Nucl Med. 1987;12(3):223-225.
- Lantz MM, Sziklas JJ, Spencer RP. Massive gastric dilatation secondary to small bowel obstruction. Demonstration by hepatobiliary images. Clin Nucl Med. 1994;19(5):464.
- O’Neill GT, McCreath G. An audit of biliary scintigraphy in a district general hospital (1993-1998) with special reference to the investigation of acalculous gallbladder disease. Nucl Med Commun. 2000;21(9):829-834.
- Tulchinsky M, Ciak BW, Delbeke D, et al. SNM practice guideline for hepatobiliary scintigraphy 4.0. J Nucl Med Technol. 2010;38(4):210-218.
- Gall CA, Chambers KJ. Cholecystectomy for gall bladder dyskinesia: symptom resolution and satisfaction in a rural surgical practice. ANZ journal of surgery. 2002;72(10):731-734.
- Tulchinsky M, Allen TW, Winner LS, Lokhandwala P, Lehman E. One hour delayed static views are sufficient prior to sincalide stimulation in patients with chronic abdominal pain referred for gallbladder ejection fraction. J Nucl Med. 2013;54(Supp 2):1944.
- Cozzolino HJ, Goldstein F, Greening RR, Wirts CW. The Cystic Duct Syndrome. Jama. 1963;185:920-924.
- Nathan MH, Newman A, McFarland J, Murray DJ. Cholecystokinin cholecystography. Radiology. 1969;93(1):1-8.
- Valberg LS, Jabbari M, Kerr JW, Curtis AC, Ramchand S, Prentice RS. Biliary pain in young women in the absnece of gallstones. Gastroenterology. 1971;60(6):1020-1026.
- Dunn FH, Christensen ED, Reynolds J, Jones V, Fordtran JS. Cholecystokinin cholecystography. Controlled evaluation in the diagnosis and management of patients with possible acalculous gallbladder disease. Jama. 1974;228(8):997-1003.
- Corazziari E, Shaffer EA, Hogan WJ, Sherman S, Toouli J. Functional disorders of the biliary tract and pancreas. Gut. 1999;45 Suppl 2:II48-54.
- Yap L, Wycherley AG, Morphett AD, Toouli J. Acalculous biliary pain: cholecystectomy alleviates symptoms in patients with abnormal cholescintigraphy. Gastroenterology. 1991;101(3):786-793.
- Raduns K, McGahan JP, Beal S. Cholecystokinin sonography: lack of utility in diagnosis of acute acalculous cholecystitis. Radiology. 1990;175:463-466.
- Moody FG, Calabuig R, Li YF, Harari Y, Rodriguez LF, Weisbrodt NW. Biliary and gut function following shock. J Trauma. 1990;30(12 Suppl):S179-184.
- Ziessman HA. Cholecystokinin cholescintigraphy: clinical indications and proper methodology. Radiol Clin North Am. 2001;39(5):997-1006, ix.
- Garrigues V, Ponce J, Cano C, et al. Effect of selective and nonselective muscarinic blockade on cholecystokinin-induced gallbladder emptying in man. Dig Dis Sci. 1992;37(1):101-104.
- Llamas-Elvira JM, Sopena R, Martinez-Paredes M, et al. Muscarinic control of gallbladder dynamics. A study using 99Tcm-HIDA and cholinergic agonists and antagonists. Nucl Med Commun. 1990; 11(11):813-817.
- Malagelada JR, Holtermuller KH, Sizemore GW, Go VL. The influence of hypercalcemia on basal and cholecystokinin-stimulated pancreatic, gallbladder, and gastric functions in man. Gastroenterology. 1976;71(3):405-408.
- Catnach SM, Fairclough PD, Trembath RC, et al. Effect of oral erythromycin on gallbladder motility in normal subjects and subjects with gallstones. Gastroenterology. 1992;102(6):2071-2076.
- O’Donnell LJ, Wilson P, Guest P, et al. Indomethacin and postprandial gallbladder emptying. Lancet. 1992;339(8788):269-271.
- Masclee AA, Jansen JB, Driessen WM, Geuskens LM, Lamers CB. Effect of truncal vagotomy on cholecystokinin release, gallbladder contraction, and gallbladder sensitivity to cholecystokinin in humans. Gastroenterology. 1990;98(5 Pt 1):1338-1344.
- Krishnamurthy S, Krishnamurthy GT. Effect of sequential administration of an opioid and cholecystokinin on gallbladder ejection fraction: brief communication. J Nucl Med. 2006;47(9):1463-1466.
- Cullen JJ, Maes EB, Aggrawal S, Conklin JL, Ephgrave KS, Mitros FA. Effect of endotoxin on opossum gallbladder motility: a model of acalculous cholecystitis. Ann Surg. 2000;232(2):202-207.
- de Boer SY, Masclee AA, Lam WF, et al. Effect of hyperglycaemia on gallbladder motility in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1994;37(1):75-81.
- Ewins DL, Javaid A, Coskeran PB, et al. Assessment of gall bladder dynamics, cholecystokinin release and the development of gallstones during octreotide therapy for acromegaly. Q J Med. 1992;83(300):295-306.
- Fisher RS, Rock E, Levin G, Malmud L. Effects of somatostatin on gallbladder emptying. Gastroenterology. 1987;92(4):885-890.
- Grimaldi C, Darcourt J, Harris AG, Lebot E, Lapalus F, Delmont J. Cholescintigraphic study of effect of somatostatin analog, octreotide, on bile secretion and gallbladder emptying in normal subjects. Dig Dis Sci. 1993;38(9):1718-1721.
- Hopman WP, Van Liessum PA, Pieters GF, et al. Postprandial gallbladder motility and plasma cholecystokinin at regular time intervals after injection of octreotide in acromegalics on long-term treatment. Dig Dis Sci. 1992;37(11):1685-1690.
- Stolk MF, van Erpecum KJ, Koppeschaar HP, et al. Postprandial gall bladder motility and hormone release during intermittent and continuous subcutaneous octreotide treatment in acromegaly. Gut. 1993;34(6):808-813.
- Mitsukawa T, Takemura J, Ohgo S, et al. Gallbladder function and plasma cholecystokinin levels in diabetes mellitus. Am J Gastroenterol. 1990;85:981-985.
- Fong YC, Hsu HC, Sun SS, Kao A, Lin CC, Lee CC. Impaired gallbladder function in spinal cord injury on quantitative Tc-99m DISIDA cholescintigraphy. Abdom Imaging. 2003;28(1):87-91.
- Annese V, Caruso N, Accadia L, et al. Gallbladder function and gastric liquid emptying in achalasia. Dig Dis Sci. 1991;36(8):1116-1120.
- Sood GK, Baijal SS, Lahoti D, Broor SL. Abnormal gallbladder function in patients with irritable bowel syndrome. Am J Gastroenterol. 1993;88(9):1387-1390.
- Kao CH, Hsieh JF, Tsai SC, Ho YJ, Chen SD. Evidence of impaired gallbladder function in patients with liver cirrhosis by quantitative radionuclide cholescintigraphy. Am J Gastroenterol. 2000;95(5):1301-1304.
- Tierney S, Nakeeb A, Wong O, et al. Progesterone alters biliary flow dynamics. Ann Surg. 1999; 229(2):205-209.
- Dumont RC, Caniano DA. Hypokinetic gallbladder disease: a cause of chronic abdominal pain in children and adolescents. J Pediatr Surg. 1999;34:858-862.
- Goncalves RM, Harris JA, Rivera DE. Biliary dyskinesia: natural history and surgical results. Am Surg. 1998;64(6):493-497; discussion 497-498.
- Canfield AJ, Hetz SP, Schriver JP, et al. Biliary dyskinesia: a study of more than 200 patients and review of the literature. J Gastrointest Surg. 1998; 2(5):443-448.
- Khosla R, Singh A, Miedema BW, Marshall JB. Cholecystectomy alleviates acalculous biliary pain in patients with a reduced gallbladder ejection fraction. South Med J. 1997;90(11):1087-1090.
- Klieger PS, O’Mara RE. The clinical utility of quantitative cholescintigraphy: the significance of gallbladder dysfunction. Clin Nucl Med. 1998;23(5):278-282.
- Middleton GW, Williams JH. Is gall bladder ejection fraction a reliable predictor of acalculous gall bladder disease? Nucl Med Commun. 1992;13:894-896.
- Pickleman J, Peiss RL, Henkin R, Salo B, Nagel P. The role of sincalide cholescintigraphy in the evaluation of patients with acalculus gallbladder disease. Arch Surg. 1985;120(6):693-697.
- Sorenson MK, Fancher S, Lang NP, Eidt JF, Broadwater JR. Abnormal gallbladder nuclear ejection fraction predicts success of cholecystectomy in patients with biliary dyskinesia. Am J Surg. 1993;166(6):672-674; discussion 674-675.
- Zech ER, Simmons LB, Kendrick RR, et al. Cholecystokinin enhanced hepatobiliary scanning with ejection fraction calculation as an indicator of disease of the gallbladder. Surg Gynecol Obstet. 1991;172(1):21-24.
- Adams DB, Tarnasky PR, Hawes RH, et al. Outcome after laparoscopic cholecystectomy for chronic acalculous cholecystitis. Am Surg. 1998;64(1):1-6.
- Mishkind MT, Pruitt RF, Bambini DA, et al. Effectiveness of cholecystokinin-stimulated cholescintigraphy in the diagnosis and treatment of acalculous gallbladder disease. Am Surg. 1997;63(9):769-774.
- Schirmer BD, Winters KL, Edlich RF. Cholelithiasis and cholecystitis. J Long Term Eff Med Implants. 2005;15(3):329-338.
- Krishnamurthy GT, Krishnamurthy S. Extended application of 99mTc-mebrofenin cholescintigraphy with cholecystokinin in the evaluation of abdominal pain of hepatobiliary and gastrointestinal origin. Nucl Med Commun. 2010;31(5):346-354.
- Szarszewski A, Korzon M, Kaminska B, Lass P. Duodenogastric reflux: clinical and therapeutic aspects. Arch Dis Child. 1999;81(1):16-20.
Citation
Tulchinsky M. Applied hepatobiliary scintigraphy in chronic gallbladder diseases. Appl Radiol. 2016;(9):16-25.
September 5, 2016