An overview of breast MRI
Images
The most commonly diagnosed cancer in women in the United States is breast cancer, and it remains a leading cause of cancer-related deaths in women aged 40 to 49.1 Currently, the American Cancer Society (ACS) recommends yearly mammography starting at the age of 40 coupled with a yearly clinical breast examination.
Overall, mammography is considered the single most effective screening tool and has been credited with reducing breast cancer-related mortality by 20-30% .2 There is no doubt that mammography is a very cost-effective tool for breast cancer screening; however, the sensitivity (67.8%) and specificity (75%) are less than ideal.3 In addition, the use of ionizing radiation and lower spatial resolution (compared to MRI) are viewed as disadvantages in younger women and in women with dense breasts.
Coupled with mammography, the use of ultrasound has become the norm when working up a mammographically or clinically suspicious lesion. However, it is a limited tool when used for screening. The most obvious disadvantages are ultrasound’s inability to detect microcalcifications and the modality’s heavy reliance on operator skill.4 Ultrasound can be used for screening with mammography in special cases, such as when an anxious patient requests ultrasound. It is important to note that at this time the ACS does not recommend using ultrasound instead of mammography for screening purposes.
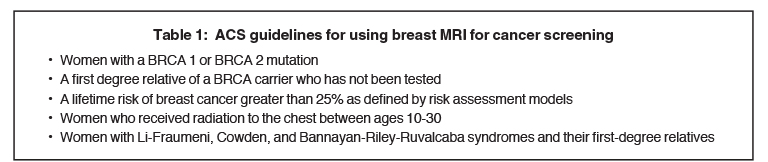
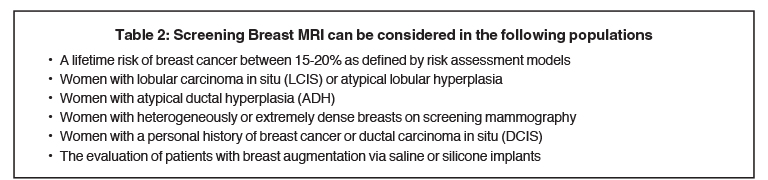
When exploring the topic of screening younger women (under 40 years), women with dense breasts, and women at high risk, one must understand the role breast MRI can play in these populations. The current ACS guidelines for using or considering the use of screening breast MRI5 are shown in Tables 1 and 2.
MRI affords the radiologist unique advantages over mammography and ultrasound. The first and foremost is the lack of ionizing radiation. Additional benefits include: better 3D spatial resolution, the ability to detect occult, multifocal/multicentric disease, better ability to delineate the true size of a cancer (often underestimated on mammography and US), and the ability to image both breasts and the chest wall.6-8
A complete evaluation of tumor involvement requires the use of MRI, as it is the best method for determining pectoralis muscle and chest wall involvement. Tumor involvement in the pectoralis muscle is seen as enhancement. The enhancing area may be surgically removed at the time of mastectomy to achieve local control, however, when the underlying serratus anterior muscles or ribs are involved (enhancing), the patient is considered to have distant metastases (T4a).
Breast MRI is not without disadvantages. The heightened sensitivity or MRI when compared to mammography (99% vs. 67.8%) is balanced by the wide range in specificity (37-97%). The main reason for this broad specificity range is that both benign and malignant lesions enhance.9, 10 MRI has a positive predictive value (PPV) much lower for areas of non- mass like enhancement. This adds to the diagnostic challenge for the radiologist. Non-mass like enhancement is associated with physiologic enhancement, fibrocystic change, benign conditions, DCIS or invasive carcinoma.11 Furthermore, MRI is expensive, requires the use of intravenous contrast, and is limited in its use in patients who cannot lie prone, are obese (over the weight limit of the scanner being used), have extremely large breasts, and are claustrophobic.8-10
Breast MRI protocol
Prior to the procedure, the patient should be interviewed and screened for contraindications to MRI. In addition, if possible, women should be scheduled for screening breast MRI during the second week of their menstrual cycle to minimize background enhancement.12
Equipment and important sequences
Typically breast MRI is performed on a 1.5 Tesla magnet with a dedicated multichannel breast coil. To detect abnormalities, achieving high spatial resolution and contrast is important. Scanning slice thickness should be 3 mm or less and resolution should be 1 mm or less in order to minimize volume averaging. MRI offers the advantage of 3D imaging, which is better at detecting smaller lesions than 2D imaging because 2D imaging produces gaps between slices in which a small lesion may be missed. To optimize contrast between a tumor and surrounding breast parenchyma, technique must be excellent. This includes proper patient positioning, protocols designed to answer the clinical question at hand, and software that dutifully performs excellent fat suppression and preserves signal-to-noise ratio.
Fat suppression is important in contrast-enhanced imaging of the breast because lesion identification in breast MRI relies upon the subtraction of pre- and postcontrast images. In pre-contrast T1 images, the bright, fatty signal from the breast can lead to mis- or overdiagnosis.13 By canceling out the fat signal within the breast and then using subtraction images, the ability to identify enhancing lesions becomes more accurate.
Our current protocol
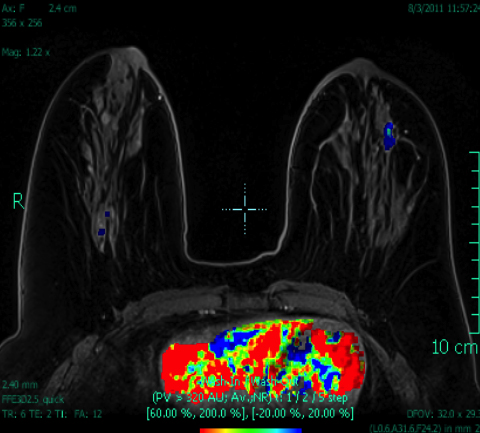
The standard protocol for a contrast-enhanced breast MRI at the authors’ institution, like most others (unless there is a contraindication), begins with the placement of an IV for the administration of gadolinium. Contrast is administered at a bolus of 0.1 mmol/kg with a power injector at a rate of 3 mL per second and flushed with 10 cc of saline.14 The patient is then placed prone into the breast coil and supported so that the breasts hang in a dependent position, which helps minimize respiratory motion and lets gravity separate the breasts. The sequences routinely obtained are shown in Table 3. Postprocessing allows the generation of the maximum intensity projection (MIP), digital subtraction sequences, and the kinetic curves.
It is important to understand the use of each sequence being generated for the examination. At our institution, the search pattern is taught to the residents and fellows in a regimented pattern. The initial sequence evaluated is the MIP as it provides a 3D overview of both breasts and enhancing/suspicious masses are easily detected. The MIP should be used as a basic overview and not relied on solely to interpret the images as it excludes non-enhancing lesions. After the MIP, the axial T2 images are reviewed, as these are fluid sensitive and are excellent for finding cysts, fluid, and inflammation. The T2 images also provide the best evaluation of the breast anatomy and skin.
Finally, the pre- and postcontrast axial T1 images are reviewed. These images are based around the same principles as digital subtraction angiography. Fat does not enhance on post-contrast sequences and using subtraction essentially cancels out all of the bright fat signal on the T1 images leaving behind the enhancing areas i.e. masses, lymph nodes and blood vessels. The T1 images are also excellent for evaluating for intraductal blood and lymph node morphology; identifying the benign fatty hilum of normal lymph nodes.
Discussion
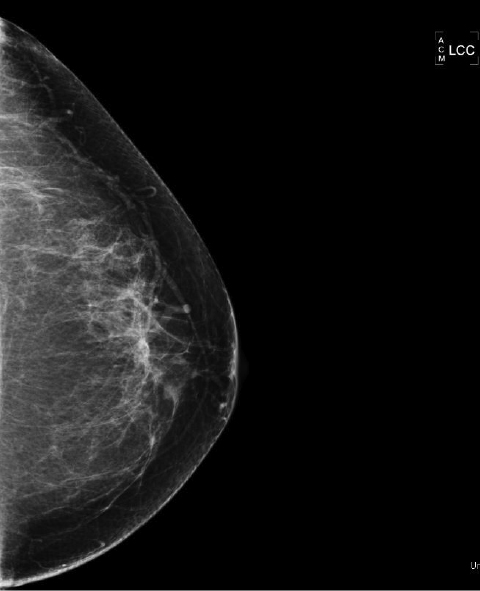
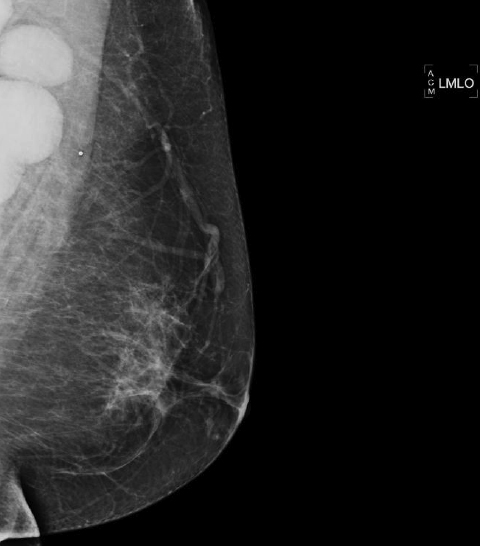
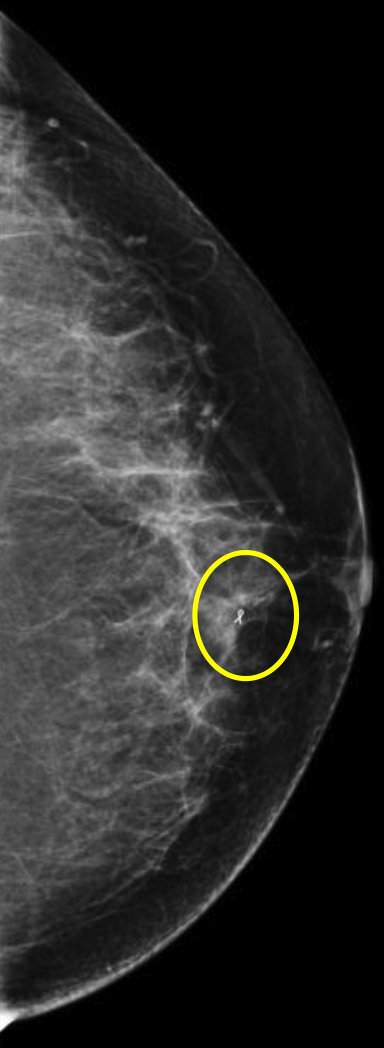
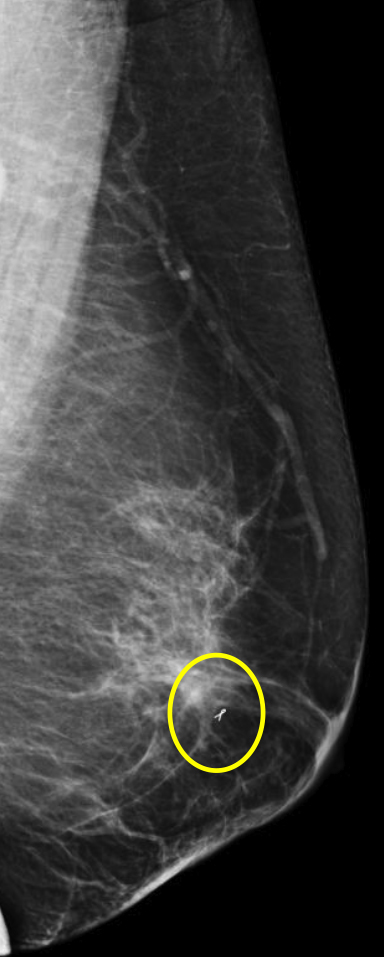
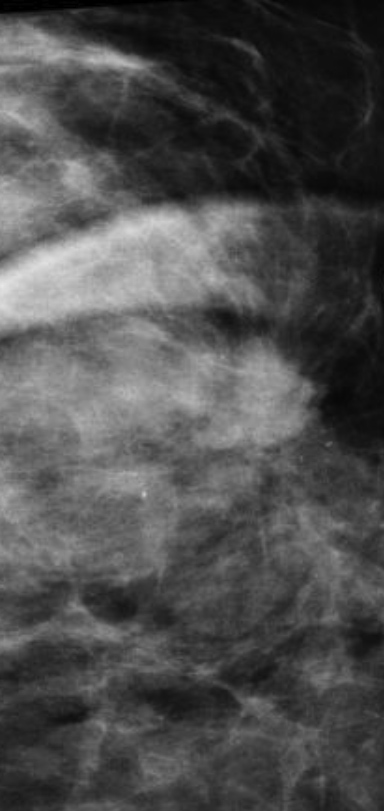
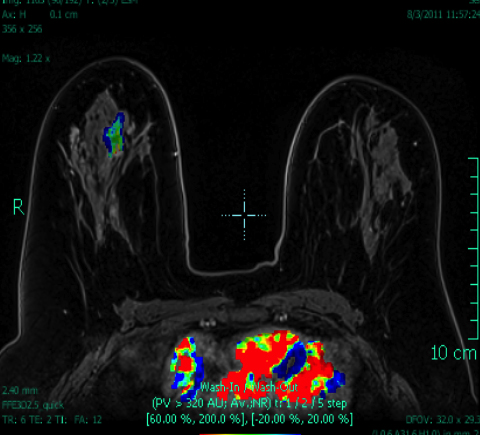
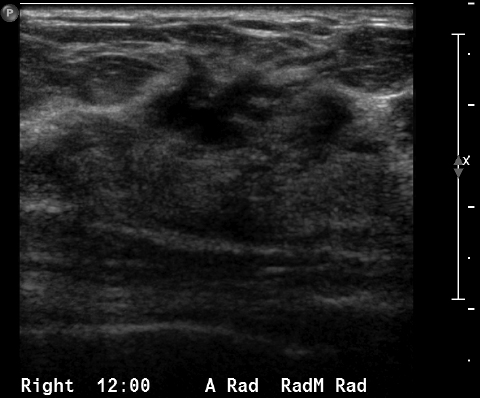
Breast MRI is a powerful tool for the radiologist when confronted with challenging clinical scenarios. Not all patients will fall neatly into the criteria mentioned above for MRI. In rare cases women will present with clinically significant axillary lymphadenopathy; however, a primary lesion cannot be found with mammography, ultrasound, or physical examination (Figure 1).
Occult primary breast carcinoma presenting as axillary lymphadenopathy accounts for less than 1% of all breast cancers.15 MRI has been found to detect 64-75% of occult breast malignancies, with the average tumor size being 15 mm at detection.16 In a study by Hong et al, MRI was found to have a low false-negative rate. No breast cancer was found in the mastectomy specimens that had negative MRI studies.17
The standard management for occult breast carcinoma is mastectomy or upper outer quadrantectomy. However, given the high sensitivity of breast MRI coupled with the low false-negative rate of lesion detection, patient management can often be altered. In their paper, Hong et al cited that as many as 35% of their reviewed patients had lesions detected at MRI and were then treated with breast conservation therapy rather than total mastectomy. The advantage of MRI over mammography and ultrasound was clearly demonstrated in this case.
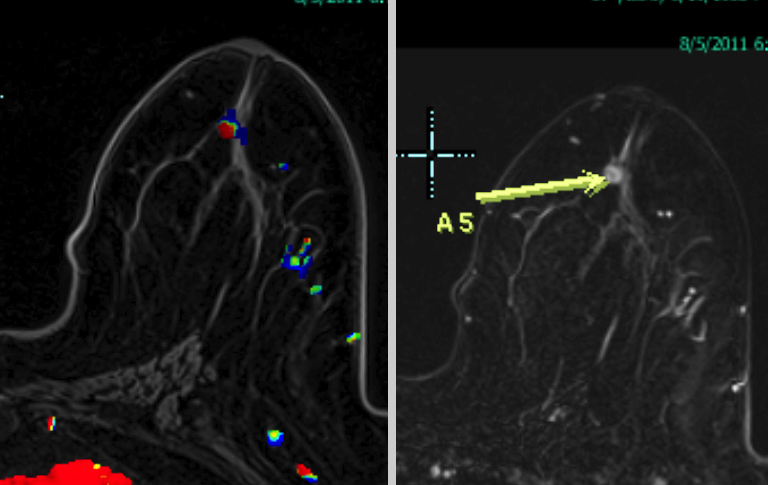
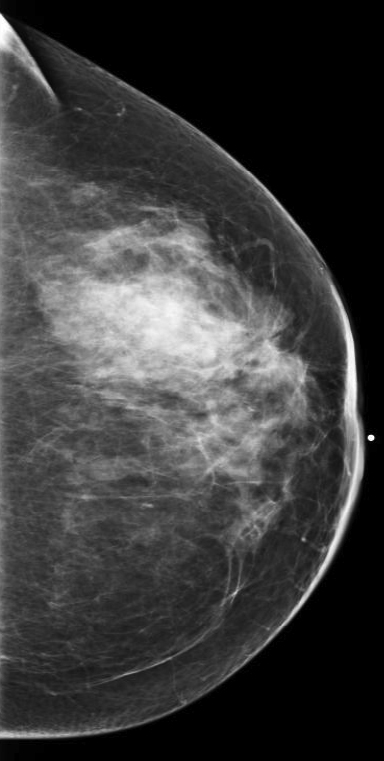
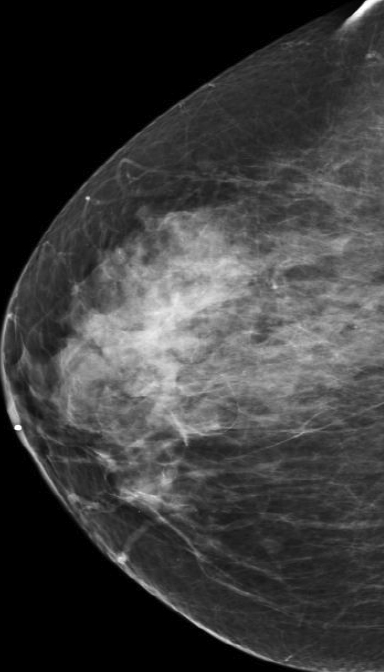
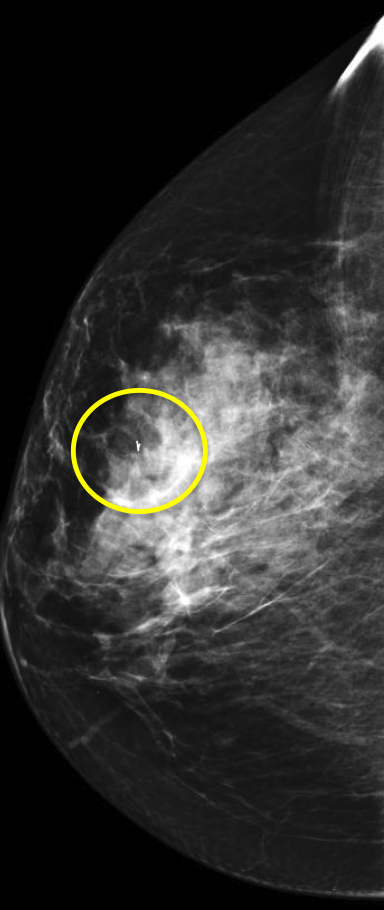
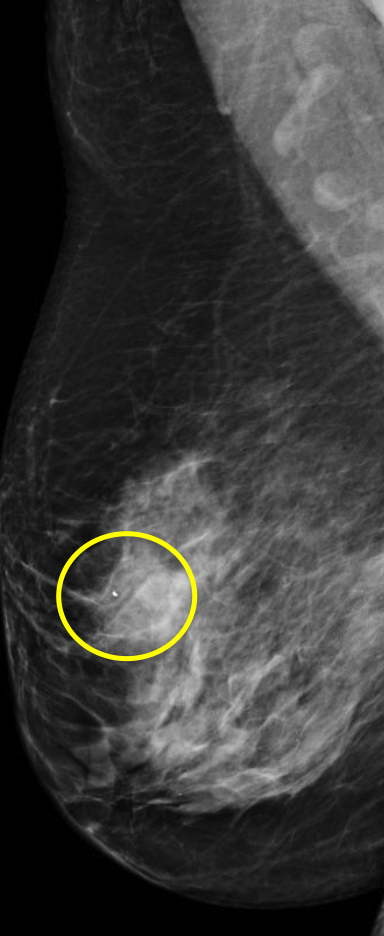
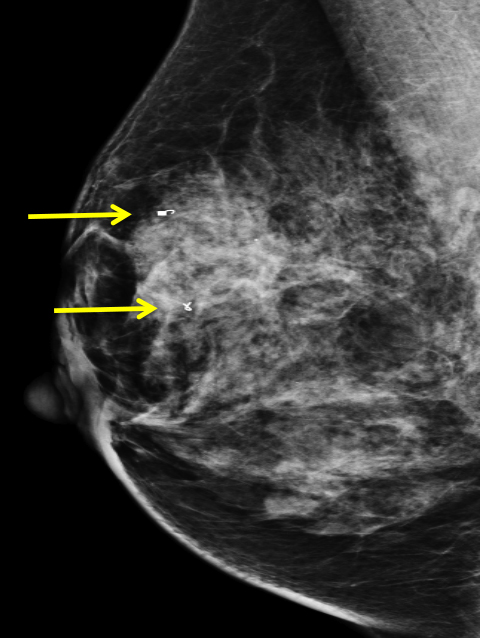
At our institution, one of the most common indications for breast MRI is preoperative evaluation of a patient with newly diagnosed malignancy (Figure 2). Patients with a history of breast cancer are at increased risk for developing a synchronous (1-3%) or a metachronous (5-7%) breast cancer in the ipsilateral or contralateral breast. Cancer detection in the contralateral breast is routinely done with mammography or physical examination. However, the sensitivities of these two methods are low; 1-3% for mammography and < 1% for physical examination.18,19 The sensitivity of MRI for detecting breast masses approaches 100% as discussed earlier with the added benefit of determining chest wall and pectoralis muscle involvement. In a study by Lee et al, all seven of the contralateral breast cancers (7 of 182) were mammographically and clinically occult. The detection of contralateral breast cancer holds prognostic significance. Patients with synchronous bilateral breast cancer or metachronous cancers diagnosed within 2 years of the primary lesion had decreased survival than those with unilateral tumors.20
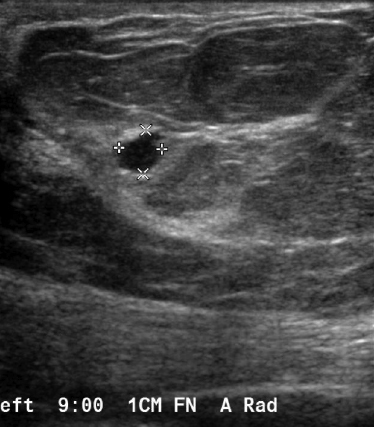
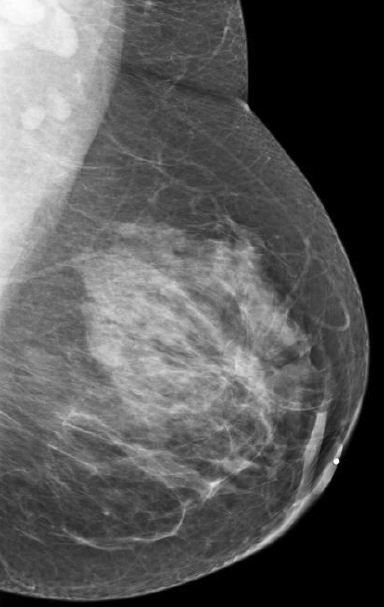
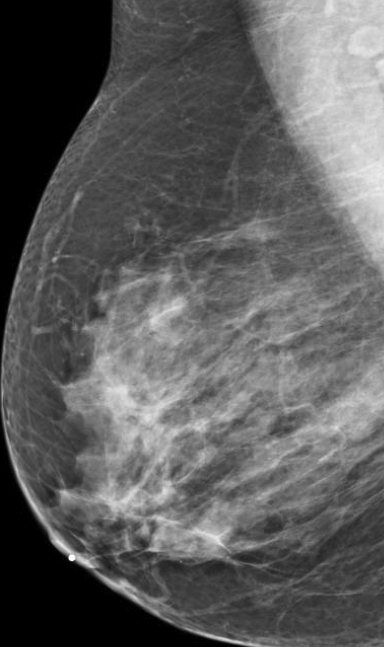
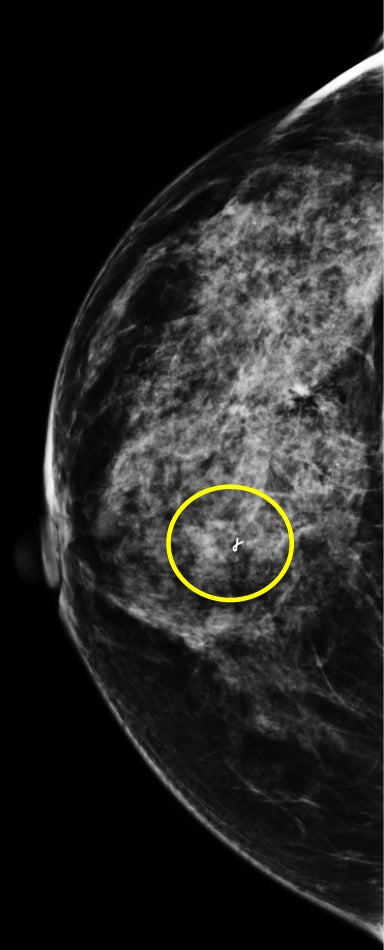
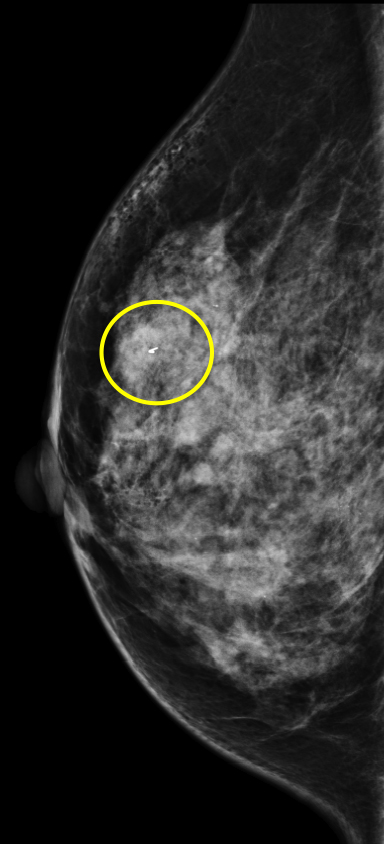
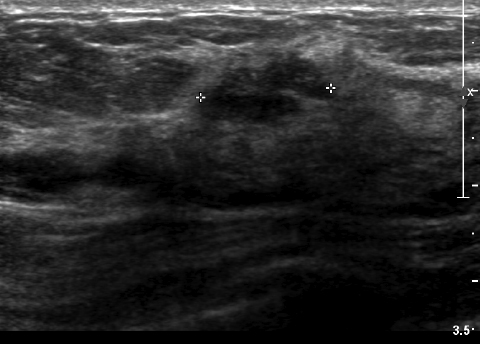
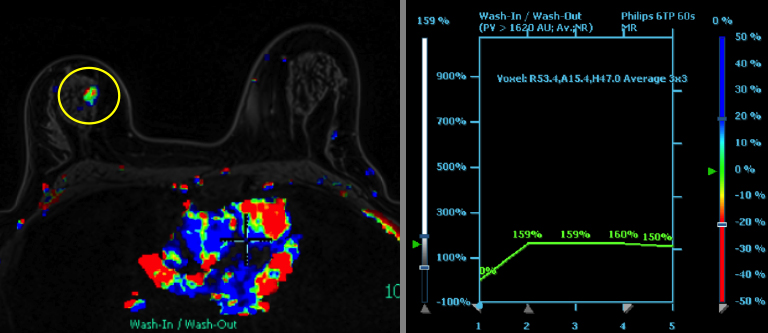
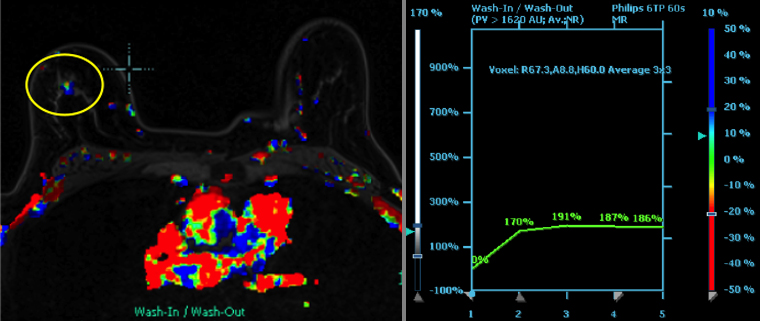
We have found that breast MRI is of particular benefit in preoperative evaluation of women with a pathologic diagnosis of invasive lobular carcinoma (ILC), as this particular entity presents as both a diagnostic and therapeutic challenge (Figure 3). It can be difficult to appreciate on physical examination because it may not form a palpable mass.21 Classic signs of architectural distortion and increased radiographic density are not common with ILC, as it does not always form a desmoplastic reaction – leading to a higher false-negative rate.22 The most common mammographic appearance of ILC is a mass with ill-defined or spiculated margins, most apparent on a single view. Ultrasound demonstrates a hypoechoic mass with posterior shadowing. The ultrasound can also demonstrate shadowing without a discernable mass. MR evaluation of ILC most often demonstrates a single, irregular mass with spiculated margins.21-23
Adding to the diagnostic challenges is the propensity for ILC to be bilateral and multicentric 20-30% of the time. In one study, further evaluation with MRI was shown to find additional foci of cancer in 13% of patients diagnosed with ILC at biopsy who were anticipating breast conservation therapy.23 In the same study, 16% of patients had a change in surgical management based on MRI findings. A meta-analysis performed by Mann et al demonstrated additional ipsilateral lesions in 32% of patients which were not seen on mammography or ultrasound.22-24 Therefore, we can conclude that MRI is a useful tool for complete evaluation and development of an optimal treatment plan for these patients.
Conclusions
Current recommendations for screening breast MRI include patients with a strong family history, BRCA 1 and BRCA 2 mutations, those with syndromes such as Cowden or LiFreumani, and those who have had radiation to the chest between the ages of 10 and 30. Breast MRI is the single best imaging modality for the evaluation of silicone implants. In addition, breast MRI is used as a problem-solving tool in specific clinical situations, particularly as a novel way to evaluate patients with a new cancer diagnosis. Breast MRI can be used to evaluate the morphology of the primary lesion, detect adjacent satellite lesions and other ipsi- and contralateral lesions. All patients with a diagnosis of ILC should undergo preoperative breast MRI, as a significant number of patients are found to have additional disease, which can alter therapy. Overall, breast MRI is an invaluable tool in the diagnosis and management of breast cancer. MRI is a more sensitive examination than both mammography and ultrasound, and when used in the appropriate clinical situations, the modality can alter patient management and improve outcomes.
References
- Kesson EM, Allardice GM, George WD, et al. Effects of multidisciplinary team working on breast cancer survival: retrospective, comparative, interventional cohort study of 13,722 women. BMJ. 2012;344:e2718.
- Shapiro S. Screening: assessment of current studies. Cancer. 1994; 74:231-238.
- Qaseem A, Snow V, Sherif K, et al. Screening mammography for women 40 to 49 years of age: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2007;146(7): 511-515.
- Kolb TM, Lichy J, Newhouse JH. Occult cancer in women with dense breasts: detection with screening US—diagnostic yield and tumor characteristics. Radiology. 1998;207(1):191-199.
- Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75-89.
- Heywang SH, Hahn D, Schmidt H, et al. MR imaging of the breast using gadolinium-DTPA. J Comput Assist Tomogr. 1986;10(2):199-204.
- Lehman CD, Gatsonis C, Kuhl CK, et al. MRI evaluation of the contralateral breast in women with recently diagnosed breast cancer. N Engl J Med. 2007;356(13):1295-1303.
- DeMartini W, Lehman C. A review of current evidence-based clinical applications for breast magnetic resonance imaging. Top Magn Reson Imaging. 2008;19(3):143-150.
- Orel SG. Differentiating benign from malignant enhancing lesions identified at MR imaging of the breast: are time-signal intensity curves an accurate predictor? Radiology. 1999;211(1):5-7.
- Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211(1):101-110.
- Gutierrez RL, DeMartini WB, Eby PR, et al. BI-RADS lesion characteristics predict likelihood of malignancy in breast mri for masses but not for non-mass like enhancement. AJR Am J Roentgenol. 2009;193(4):994–1000.
- Lehman CD, Blume JD, Weatherall P, et al. Screening women at high risk for breast cancer with mammography and magnetic resonance imaging. Cancer. 2005;103(9): 1898-1905.
- Azlan CA, DiGiovanni P, Ahearn TS, et al. B1 Transmission-field inhomogeneity and enhancement ratio errors in dynamic contrast-enhanced MRI of the breast at 3T. J Magn Reson Imaging. 2010;31(1):234-239.
- ACR practice parameter for the performance of contrast enhanced MRI of the breast. http://www.acr.org/~/media/2a0eb28eb59041e2825179afb72ef624.pdf
- Stomper PC, Waddell BE, Edge SB, et al. Breast MRI in the evaluation of patients with occult primary breast carcinoma. The Breast Journal. 1999;5(4):230–234.
- De Bresser J, de Vos B, van der Ent F, et al. Breast MRI in clinically and mammographically occult breast cancer presenting with an axillary metastasis: A systematic review. Eur J Surg Oncol. 2010; 36(2):114-119.
- Lu H, Xu Y, Zhang S, et al. Breast MRI in patients with occult breast carcinoma: evaluation on feasibility and correlation with histopathologic findings. Chin Med J. 2011;124(12):1790-1795.
- Lee SG, Orel SG, Woo IJ, et al. MR imaging screening of the contralateral breast in patients with newly diagnosed breast cancer: preliminary results. Radiology. 2003; 226(3):773-778.
- Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MRI on the therapeutic approach. Radiology. 1999; 213(3):881-888.
- Kollias J, Ellis I, Elston C, et al. Prognostic significance of synchronous andmetachronous bilateral breast cancer. World J Surg. 2001:25(9): 1117-1124.
- Albayrak ZK1, Onay HK, Karatağ GY, et al. Invasive lobular carcinoma of the breast: mammographic and sonographic evaluation. Diagn Interv Radiol. 2011;17(3): 232-238.
- Lopez J, Bassett L. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics. 2009;29(1):
- 165-176.
- Berg, W, Madsen KS, Schilling K, et al. Breast cancer: Comparative effectiveness of positron emission mammography and MR imaging in presurgical planning for the ipsilateral breast. Radology. 2011;258(1):59-72.
- Mann, R, Hoogeveen YL, Blickman JG, et al. MRI compared to conventional diagnostic work-up in the detection and evaluation of invasive lobular carcinoma of the breast: a review of existing literature. Breast Cancer Res Treat. 2008;107(1):1-14.
Citation
H S, JF W, C D, KA OPK.An overview of breast MRI. Appl Radiol. 2016; (10):7-13.
October 15, 2016