Virtual Imaging Trials: The Next Big Thing?
Advances in computational resources such as software, processing, and storage have paved the way for exciting applications in the relatively new field of computational medicine, which uses data-driven methods to gain individualized insights into diseases and treatments. In recent years, expanding computational resources has been foundational to many developments in machine learning, artificial intelligence, and digital simulations, to name a few. Using representations or models, computational approaches are expected to have a major impact on research and care delivery across disciplines, particularly in radiology.
Emulating reality using representational and computational methods through virtual means is being explored as an alternative to clinical trials. In particular, representing real medical entities or processes in the form of digital twins in virtual trials is gaining ground to meet the challenges in evaluating imaging innovations.
“We need to have more proficient ways to evaluate the effectiveness of medicine,” says Ehsan Samei, PhD, Rice distinguished professor of radiology at Duke University and director of the Center for Virtual Imaging Trials (CVIT) in Durham, North Carolina. “Evaluation of medical innovations is ideally done through trials on humans, but such trials are extremely cumbersome,” he says. Barriers include costs, trial duration, ethical concerns, and diversity of subjects, among other logistical constraints.
Virtual trials provide realistic and validated simulations as an alternative approach to assessing the impact of treatments and technologies on outcomes. The models present “a very sophisticated ‘cartoon’ of the patient as complete and as realistic as we can make it,” says Dr Samei, noting that a virtual representation can be “almost and effectively as real as the real thing.”
“ In silico imaging trials are the next big revolution in radiology, which will enable us to accelerate the development of imaging systems,” says Alejandro (Alex) Frangi, PhD, Bicentennial Turing Chair in Computational Medicine at the University of Manchester, England. Not surprisingly, interest is high among clinicians, researchers, policymakers, regulatory bodies, insurance companies, and vendors.
“While virtualizing reality has been implemented in various industries and scientific pursuits, it has not been fully embraced as an essential resource in medical imaging,” says Dr Samei. To discuss research, development, and use cases surrounding the concept, more than 130 stakeholders attended the first Virtual Imaging Trials in Medicine (VITM) summit held at Duke University in April. Presentations focused on applications for virtual imaging trials in CT, digital tomosynthesis, SPECT, dental imaging, breast density estimation, imaging technique optimization, cancer care, and personalized medicine.
Technology Applications
Among benefits, creating simulations of trials enables a ground truth comparison allowing researchers and imaging scientists to optimize imaging processes and performance. Individualized cancer care is one area of application.
“[Researchers are creating] individualized models of patients’ tumors so they can predict whether the tumor is progressing or regressing,” explains Francesco Ria, PhD, assistant professor of radiology at Duke’s CVIT. “We can use this methodology to personalize care for individualized patients.”
Virtual trials can help inform the design of human trials. Benefits, which are applied in product design and development, include simplified experiments, evidence for effective medicine, and individualized patient care. Another advantage is a more cost-effective evaluation of developing technologies since ensuring that prototypes are safe and effective is an expensive, cumbersome process, says Dr Samei.
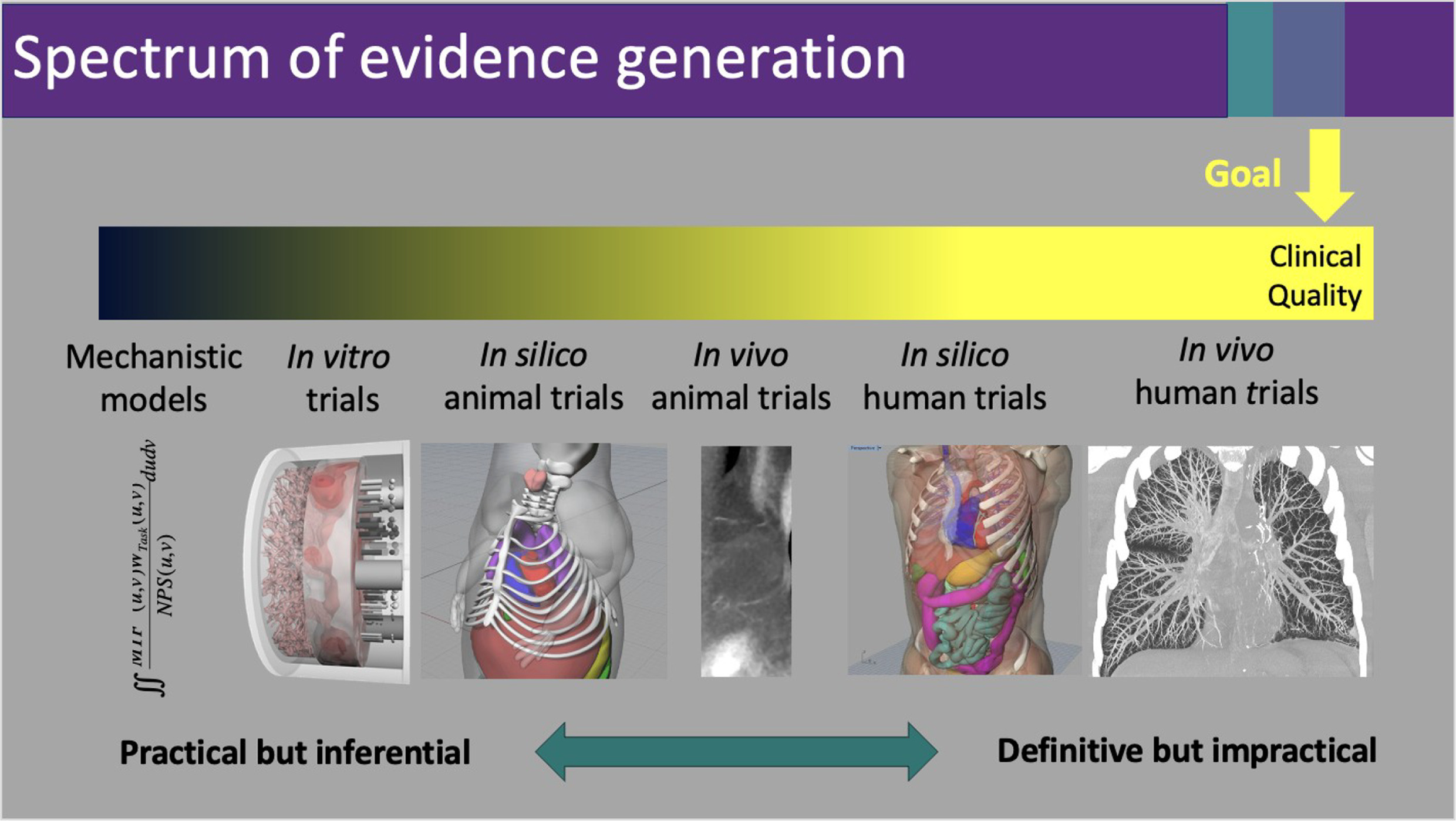
“[Vendors] need evidence to prove what they’ve produced is of high clinical quality,” says Dr Samei. This is achievable within the same continuum of practical to definitive evidence generation as shown in Figure 1 .
The left-sided metrics tend to be more practical, while those on the right tend to more closely represent the clinical reality, the ultimate goal. Graphic provided by Ehsan Samei, PhD.
For example, VITM summit poster presenter Gustavo Pacheco of Radboud University Medical Center in the Netherlands studied the optimization and validation of a compression model for realistic digital breast phantoms in mammography and digital breast tomosynthesis simulations. While breast compression is essential for achieving optimal image quality and reducing patient radiation exposure, it is necessary to pinpoint the optimal protocol and quantitatively assess the actual benefit of the procedure, which is often uncomfortable and negatively affects compliance with mammography recommendations, notes Dr Samei.
“Those questions need to be answered in the context of individual patients based on clinical effectiveness, not some back-of-envelope calculation,” he says. “No one has ever done a systematic trial [on] reducing breast compression to see what the results would be.”
“Virtual trials also enable studies on realistic populations of computational patients spanning ages, genders, and races for a wide variety of conditions, including uncommon diagnoses for which enrolling an adequate number of patients might be difficult.” In such scenarios, virtual data can supplement real data to provide more robust answers, says Dr Ria.
Consider CT imaging of patients with emphysema. When assessing treatment effectiveness, clinicians try to optimize the imaging technique to tease out the signatures of the disease in the best way possible, which is typically done as a “best guess,” says Dr Samei. By creating emphysema in virtual patients with the exact extent of their disease, the clinician can determine the utility of images to assess disease progression or regression and, thus, how to best manage each patient.
Future Directions
Another example of virtual trial potential involves volumetric measurement of organs through deep-learning, automated virtualization of CT images, the topic of a study presented at the VITM by Duke’s Mobina Ghojogh Nejad, MD. By studying organ size, virtual models enable accurate assessment of organs and, thus, anatomical progressions that are associated with certain conditions.
“If we found some correlation between organ volume and age or BMI [body mass index], we can use this data to inform our models to reflect real subjects’ changes associated with age,” says Dr Ria.
The use of VITM may also offer an approach to engineer new imaging biomarkers that “exploits our understanding of the imaging physics and disease mechanisms more fundamentally than in current biomarker discovery approaches for diagnosis and monitoring of drug effects that largely rely on trial and error,” says Dr Frangi. “These techniques could be adopted more broadly as part of the [research and development] lifecycle of imaging systems and biomarker engineering.”
For virtual trials to grow, however, tools must be democratized for all to use clinically and scientifically, says Dr Samei.
“We need to have consistent protocols, vocabulary, and ways of communicating to be able to cross-operate across resources that have been developed in multiple places, making them easily accessible and available to everyone,” he says. “We need a cohesive vision and a dedicated focus across the health care enterprise to move [virtual trials] forward.”
Citation
K, R. Virtual Imaging Trials: The Next Big Thing?. Appl Radiol. 2024;(4):36 - 38.
doi:10.37549/AR-D-24-0004
August 1, 2024