Vasculitis in the Emergency Room: The Pivotal Role of Imaging in Diagnosis and Management
Images







Abstract
Vasculitis encompasses a range of conditions characterized by blood vessel inflammation with diverse clinical presentations. Classified by vessel size, vasculitis includes large-vessel vasculitis such as giant cell arteritis, medium-vessel vasculitis like Kawasaki disease, and small-vessel vasculitis like granulomatosis with polyangiitis. Misdiagnosis can have severe consequences such as heart attack and stroke. While biopsy remains the diagnostic gold standard, imaging plays a crucial role given the challenge of tissue sampling. Imaging findings combined with clinical evidence are essential for accurate diagnosis and treatment. This article explores various vasculitis types and their clinical and imaging findings in emergency settings.
Keywords: Vasculitis, large vessel vasculitides, giant cell arteritis, Takayasu arteritis, Kawasaki Disease, granulomatosis with polyangiitis
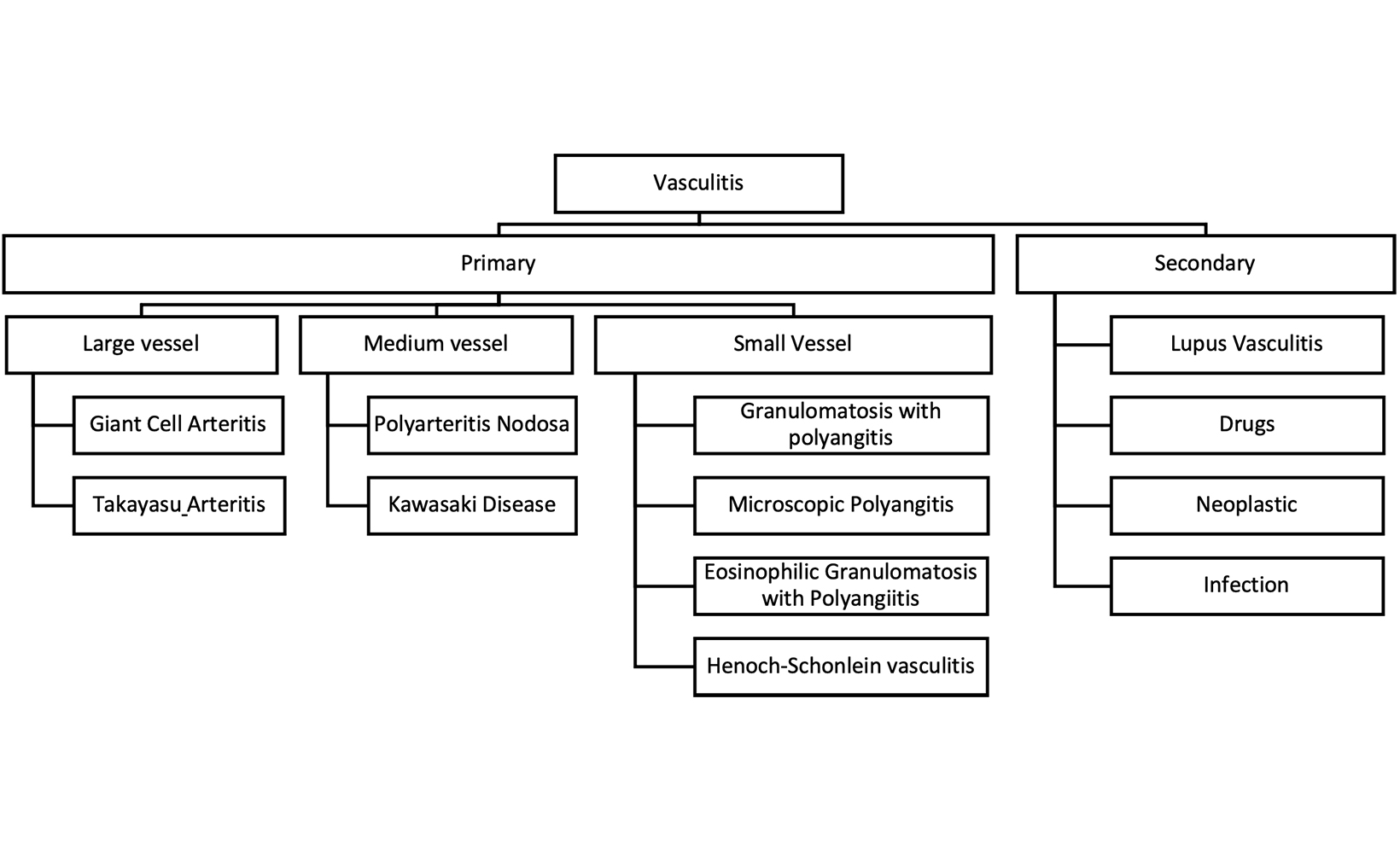
Vasculitis, encompassing a broad spectrum of disorders, is a beacon of medical intrigue, primarily characterized by inflammation within the vessels that nourish our organs 1 Vasculitis can be primary or secondary, depending on etiology. According to the 2012 revised Chapel Hill Consensus Conference Nomenclature of Vasculitides, it is intricately categorized by the size of the involved vessels into large-vessel vasculitis (eg, giant cell arteritis, Takayasu arteritis), medium-vessel vasculitis (eg, Kawasaki disease, polyarteritis nodosa), or small-vessel vasculitis (eg, granulomatosis with Polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, Henoch-Schonlein purpura, systemic lupus erythematosus, rheumatoid vasculitis, Behçet syndrome) (Figure 1).2,3 This classification framework is instrumental in comprehensively characterizing the diverse imaging manifestations of vasculitis, thus facilitating accurate diagnosis and timely management.
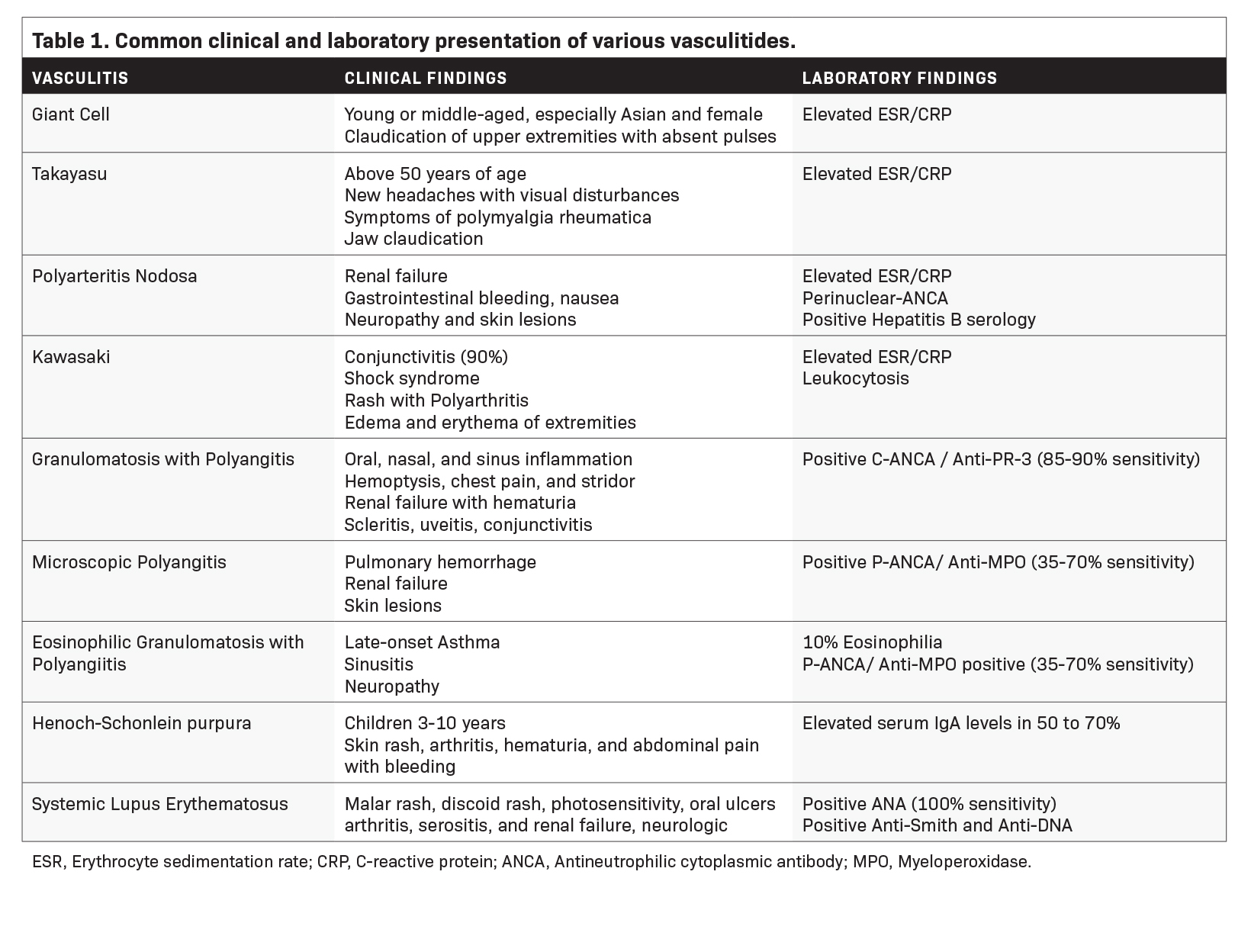
For many vasculitis patients, their initial presentation occurs in the emergency department. Misdiagnosis and delays in achieving diagnosis can lead to a risk of heightened mortality and morbidities such as myocardial infarction, stroke, mesenteric ischemia, or ruptured aneurysms. The clinical presentation of vasculitis often exhibits similarities to other medical conditions, which can contribute to misdiagnosis (Table 1). The differential diagnosis must consider the possibility of infections, coagulopathies, and drug toxicities.1,4 Although biopsy is the gold standard of diagnosis, tissue sampling can be challenging, and biopsy may be negative in up to 42% of cases.5 In this intricate maze, radiologists emerge as invaluable navigators, leveraging imaging to elucidate the causes of vasculitis. In addition, it is essential to image these patients before initiation of therapy, since the sensitivity of imaging notably decreases a few days after the initiation of corticosteroid therapy.6
A multidisciplinary approach that harnesses radiological and clinical evidence is essential to enhance diagnostic accuracy, assess disease extent, and enable better treatment selection and monitoring. 7 This article endeavors to traverse the intricate nature of vasculitis, spotlighting the confluence of clinical presentations and imaging findings, especially in the emergency department.
Large-vessel Vasculitis
Giant Cell Arteritis
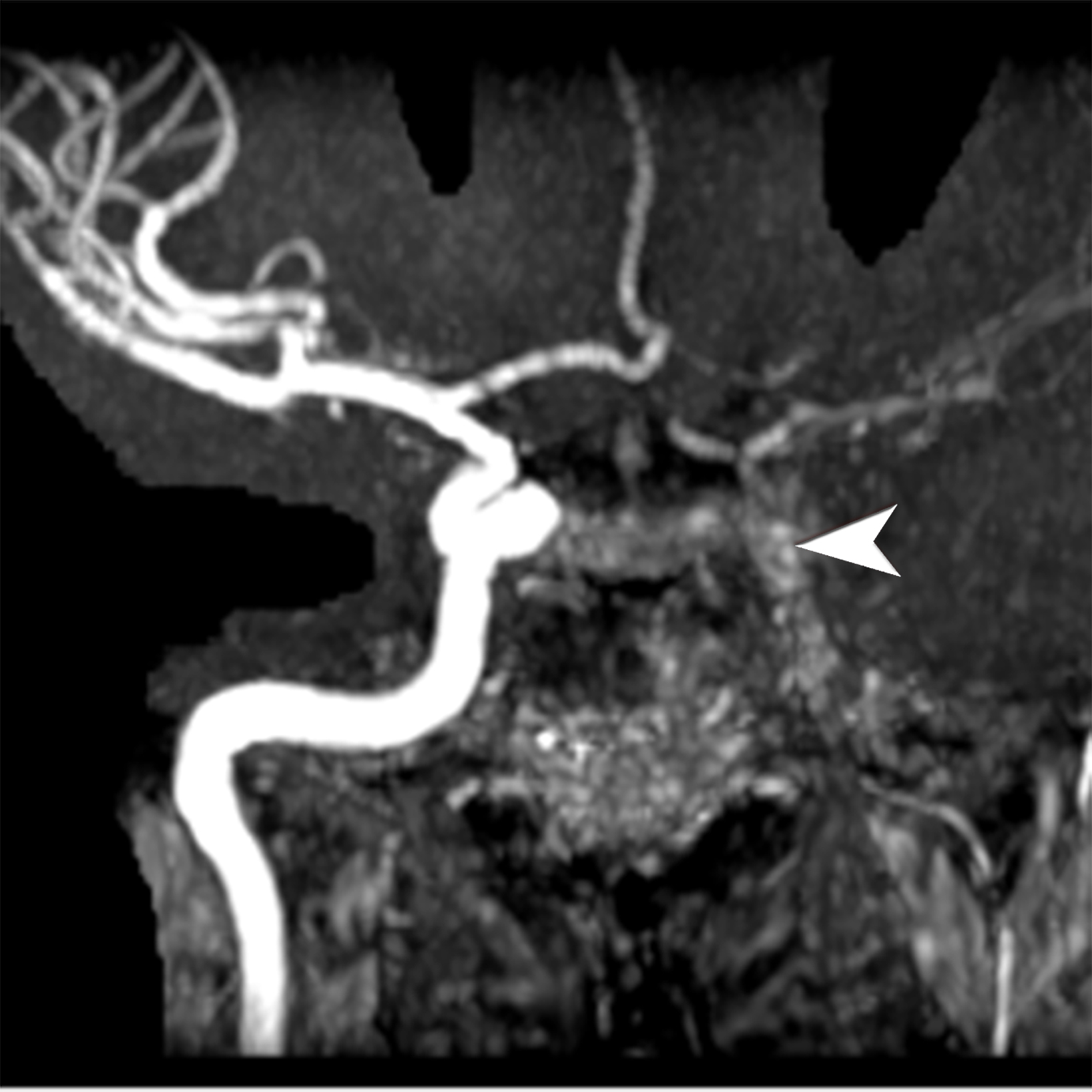
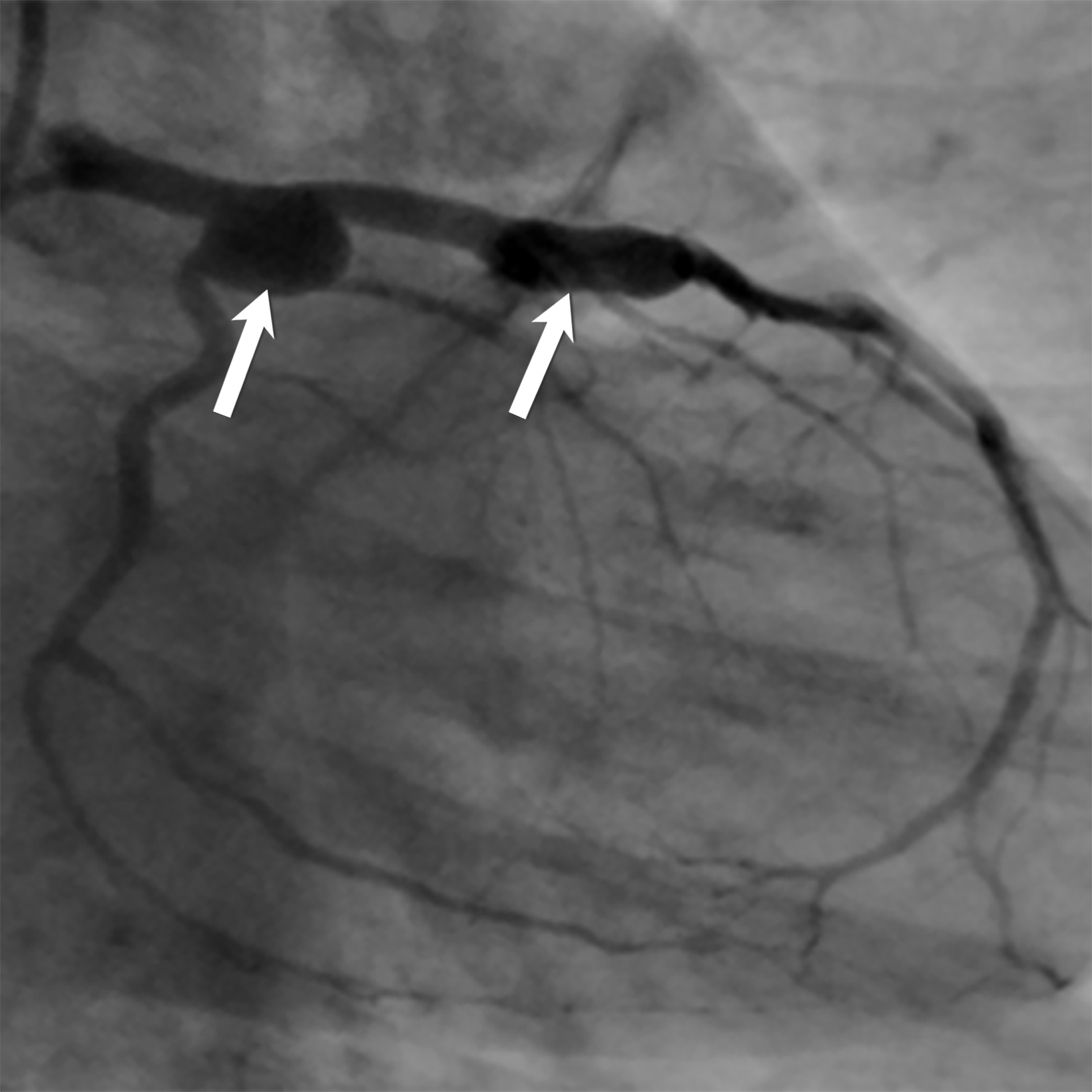
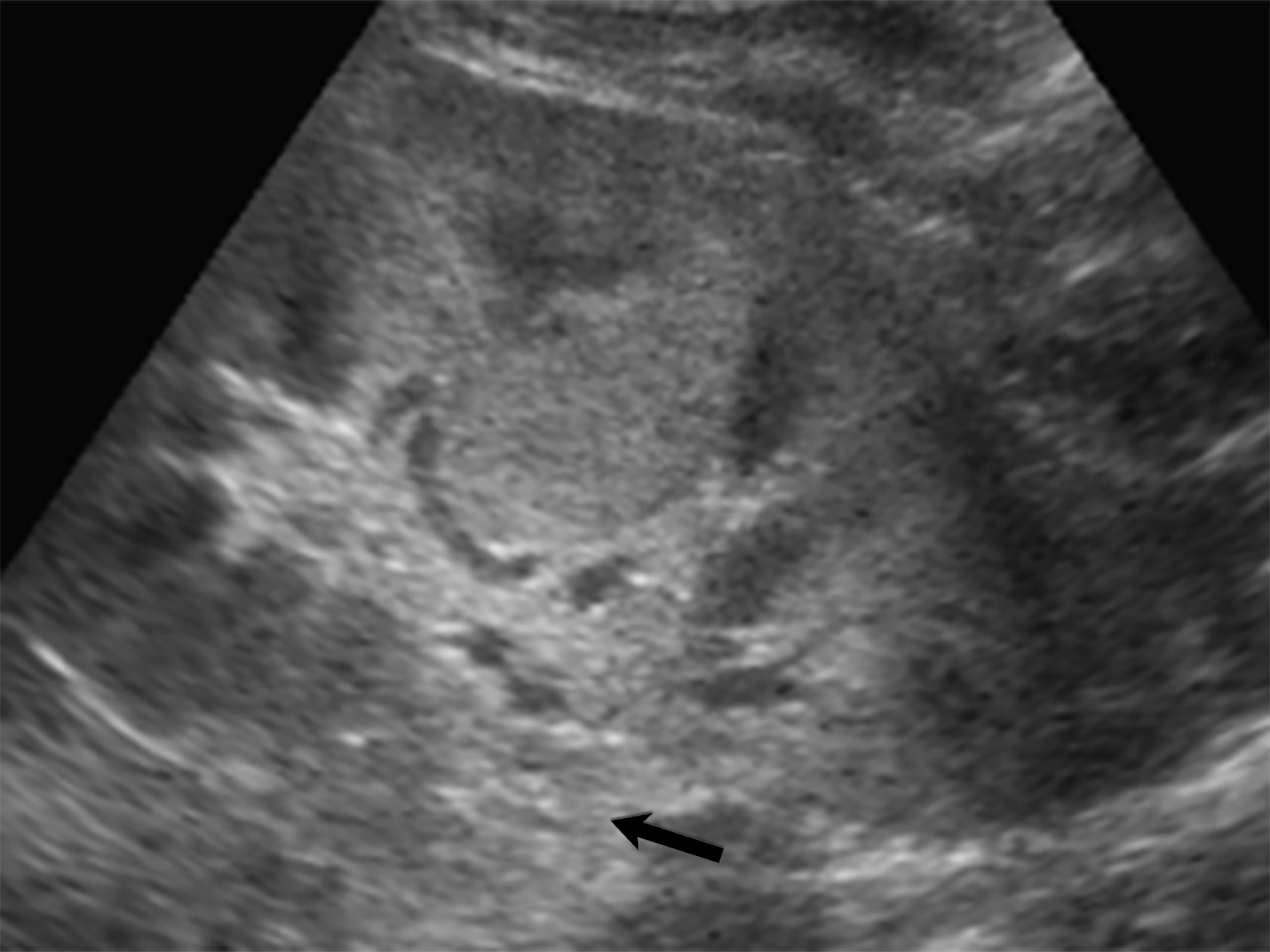
Giant cell arteritis (GCA) is an autoimmune chronic granulomatous inflammation of the large vessels that stands as the most common inflammatory vasculitides.8 It often occurs in people over 50 years of age, with a predilection for those of northern European descent.7 The primary pathology in GCA is transmural inflammation of the vessels, causing luminal occlusion, aneurysm formation, dissection, hemorrhage, and rupture 7, most commonly involving the carotid arteries and, rarely, the central pulmonary arteries.9 Key clinical presentations in the emergency setting that should trigger a heightened index of suspicion for GCA include the main clinical features outlined in the 2022 American College of Rheumatology (ACR)-European Alliance of Associations for Rheumatology (EULAR) Classification Criteria such as sudden monocular visual disturbances, new onset headache, jaw claudication, limb claudication, asymmetric blood pressure, or vascular bruits.8,10-12 Polymyalgia rheumatica is the most common extracranial manifestation in GCA, seen in 45-50% of patients who classically present with severe pain and stiffness of the shoulders, neck, and less frequently the pelvic girdle.5,8 Suspicious clinical presentation and elevated inflammatory markers should prompt a thorough diagnostic workup for GCA.
Doppler ultrasound (US) of the temporal artery reveals noncompressible hypoechoic circumferential vessel wall thickening, known as the “Halo sign.” Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) assess large-vessel involvement, demonstrating vessel wall thickening, arterial stenosis, aneurysms, or dissections (Figure 2). Delayed CTA images can help delineate vessel wall enhancement whereas noncontrast images differentiate true wall thickening from intramural hematoma, seen in acute aortic syndrome.8 In addition, magnetic resonance imaging (MRI) can accurately assess large-vessel involvement as an increased signal on fat-suppressed T2 images and late enhancement in delayed post-contrast imaging. Cardiac MRI and echocardiography can also assess valvular involvement, most commonly aortic insufficiency, coronary artery disease or, less commonly, myocarditis and pericarditis.7,13 Steady-state free precession (SSFP) cine sequence in cardiac MRI helps to assess the cardiac function and valvular involvement while delayed enhancement images accurately depict fibrosis and inflammation.7 Temporal artery biopsy is the standard but invasive diagnostic test. 11
Takayasu Arteritis
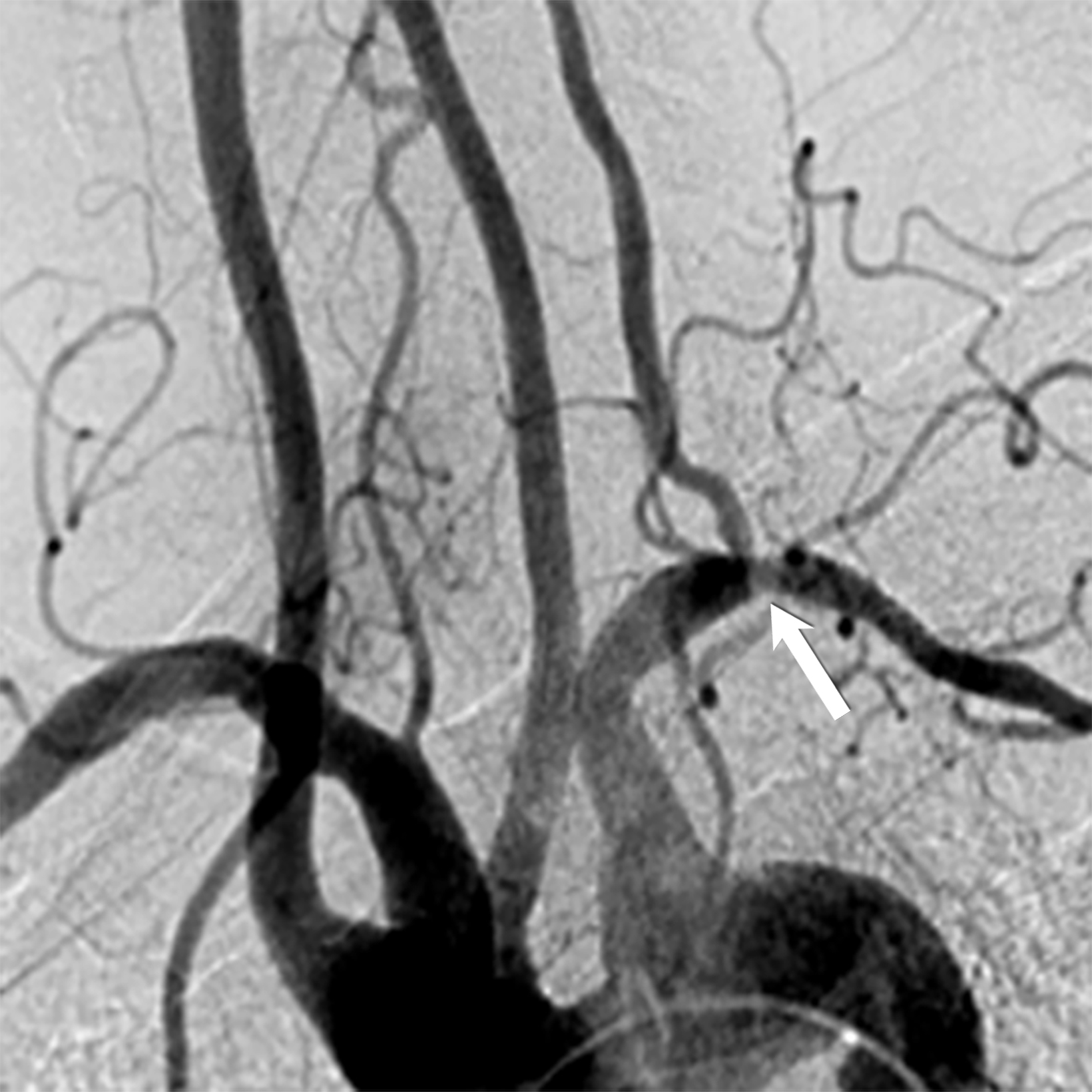
Takayasu arteritis (TAK) is a rare granulomatous large-vessel vasculitis affecting 1.11 per million people yearly. It commonly affects those younger than 50 years old, distinguishing it from GCA.6,14 TAK tends to cause panarterial inflammation, leading to luminal narrowing and occlusion,7 and can present with various clinical manifestations depending on the affected vessels, similarly to GCA.15
Early-stage TAK may present with nonspecific symptoms; as result, it is often misdiagnosed, emphasizing the importance of a high clinical suspicion index and comprehensive imaging studies. Alarming clinical presentation in the emergency department for TAK includes one of the key clinical features in the 2022 ACR-EULAR Classification Criteria such as angina, claudication, vascular bruit or systolic blood pressure difference in arms ≥ 20 mm Hg.12
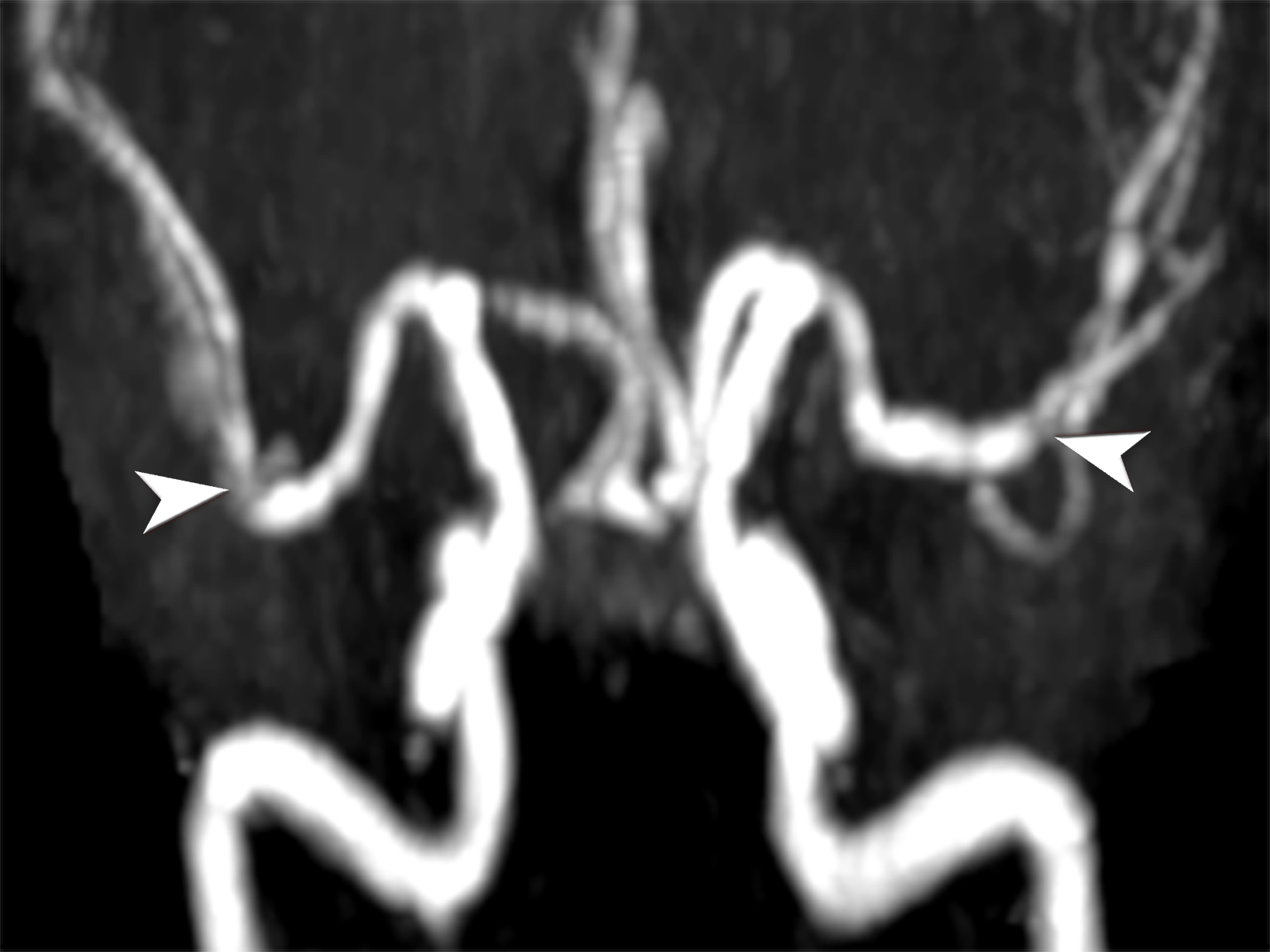
Noninvasive imaging modalities are the current standard for diagnosing and assessing disease extent. Ultrasound demonstrates intima-media thickness greater than 1mm and vascular stenosis in TAK involving the carotid and subclavian arteries. However, ultrasound is limited in assessing the aorta or major aortic branches, those most commonly involved in TAK.16 CTA and MRA are the diagnostic imaging modalities primarily used to diagnose wall thickening (low attenuation ring in delayed phase) and luminal stenosis (Figure 3).17 Using the fat-suppressed black-blood technique during contrast-enhanced MRI scans enables the identification of early inflammatory vascular changes such as mural thickening, potentially revealing reversible stages, even before there is an appreciable wall thickening.6,13 Like GCA, cardiac MRI and echocardiography can assess cardiac involvement.7 PET/CT can help determine disease activity. Conventional angiography used to be the gold standard for evaluating affected vessels; however, it is invasive and cannot assess vascular wall involvement, which occurs earlier in the disease pathogenesis.15,18
Medium-vessel Vasculitis
Kawasaki Vasculitis
Kawasaki disease (KD) is a pediatric, medium-vessel vasculitis prevalent in children under five years old. The incidence of KD is notably higher in Japan, with 265 cases per 100,000, in contrast to the 20-25 cases per 100,000 in the United States.7,19,20 Kawasaki disease is believed to stem from abnormal immune responses. The classic presentation includes fever persisting for at least five days and the presence of at least four of the following criteria: bilateral nonexudative conjunctivitis, oropharyngeal mucosal changes, erythema and desquamation of hands and feet, non-vesicular truncal rash, and cervical lymphadenopathy.7,19,20
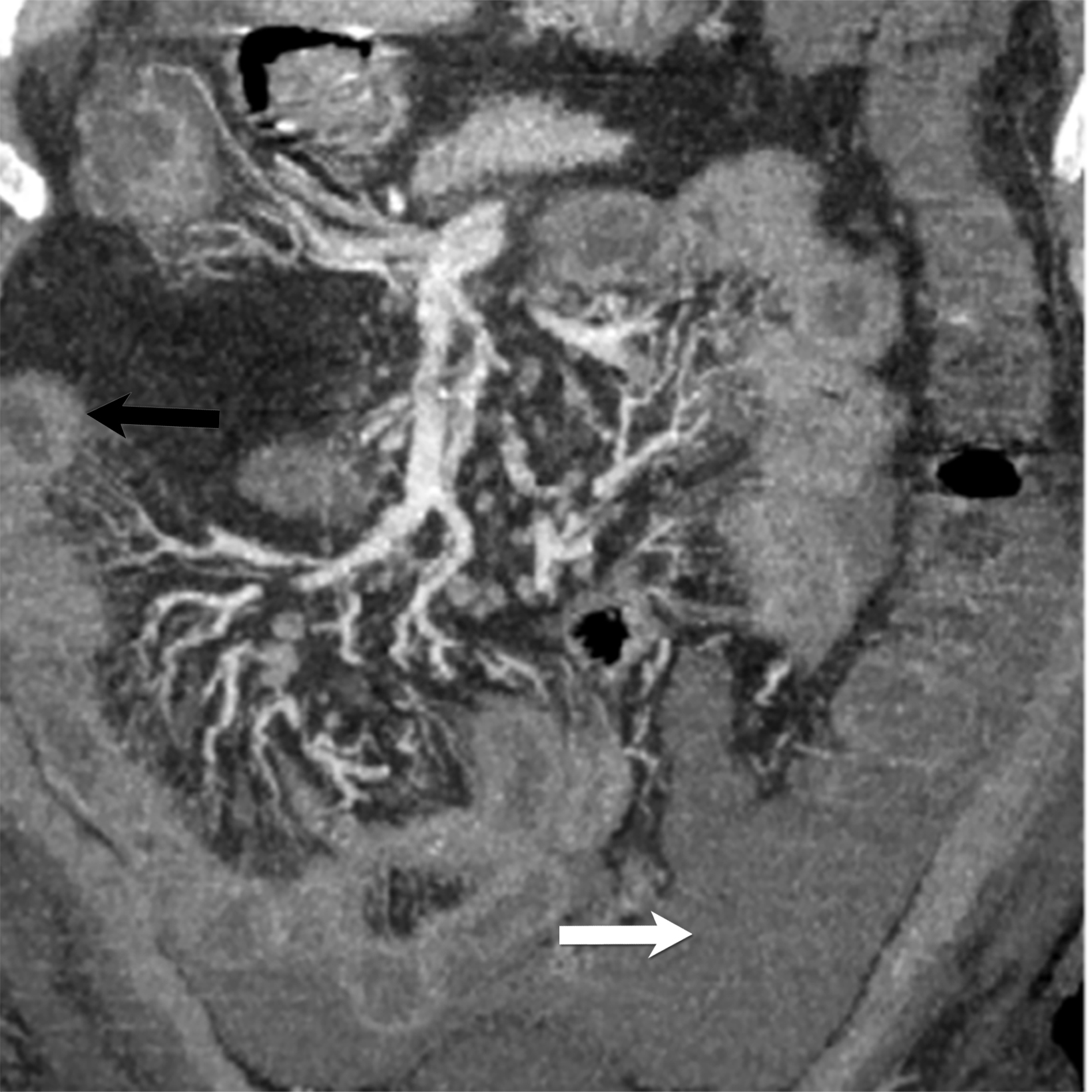
Kawasaki disease is a major contributor to acquired pediatric cardiovascular diseases, with the most common complication being coronary artery aneurysm, occurring in 15-25% of untreated cases and posing a potential risk of thrombosis and myocardial infarction.21 Additional possible complications include pericarditis, myocarditis, valvular regurgitation, and aneurysmal formation in non-coronary arteries.
Echocardiography is the preferred diagnostic modality to detect coronary aneurysms, owing to its ability to reveal accular or fusiform arterial dilatation. In chronic phases, echocardiography depicts mural thrombosis of the aneurysm as hyperechogenicity and luminal stenosis.19 Coronary CTA is a rapid modality that can accurately diagnose coronary artery stenosis in the emergency setting with negative predictive value up to 99%. However, optimal imaging techniques should be applied to lower radiation doses given most of these patients are pediatrics.21 CTA can also evaluate coronary anomalies, particularly those involving the distal arteries (Figure 4).
MRI is valuable for assessing cardiac function and myocardial viability using a three-dimensional SSFP sequence or non-enhanced black-blood MRA to assess coronary arteries involvement. 7,21,22 Patients can also develop other presentations with nonspecific imaging findings, including pseudo-obstructions, hepatitis, pancreatitis, arthritis, and myositis.19
Polyarteritis Nodosa
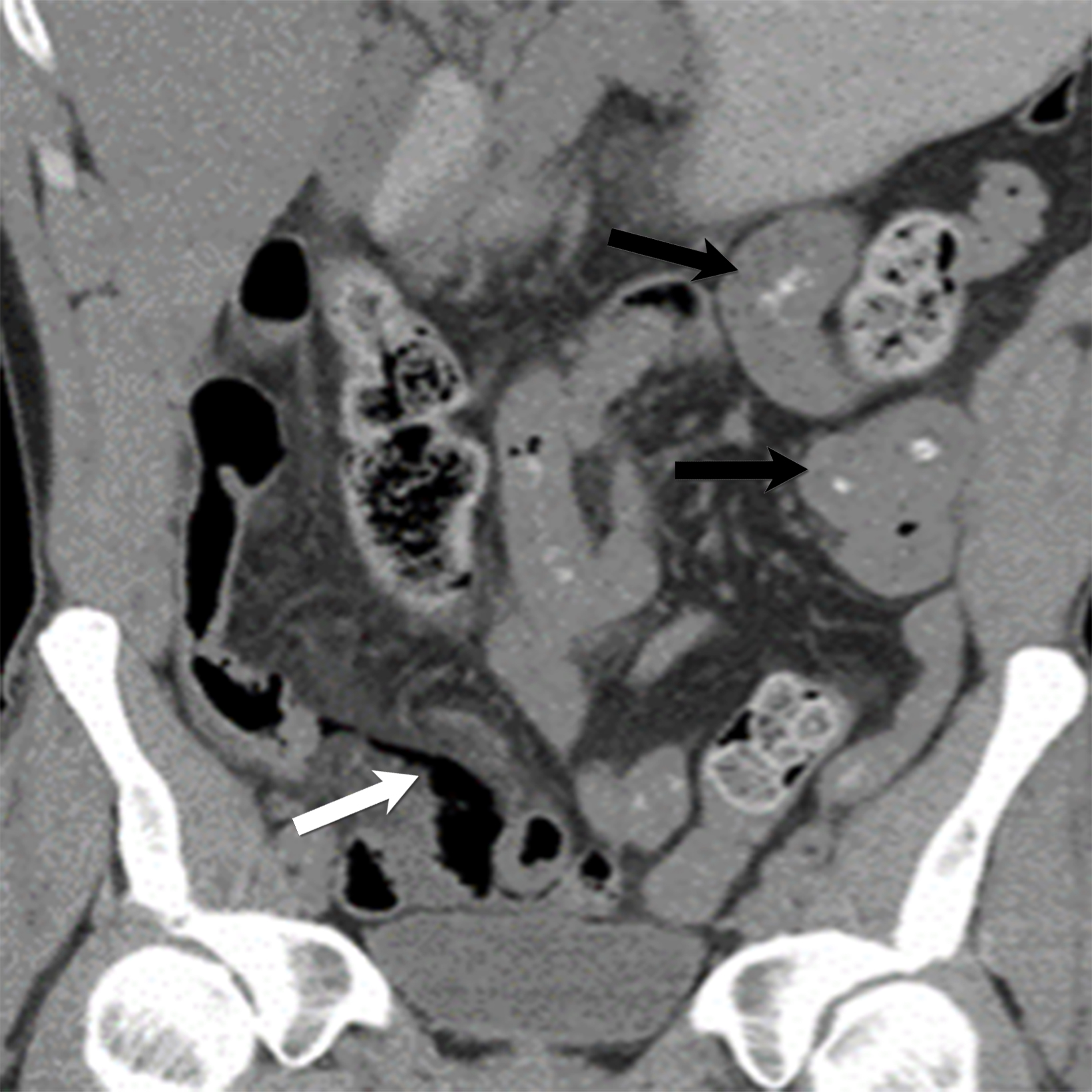
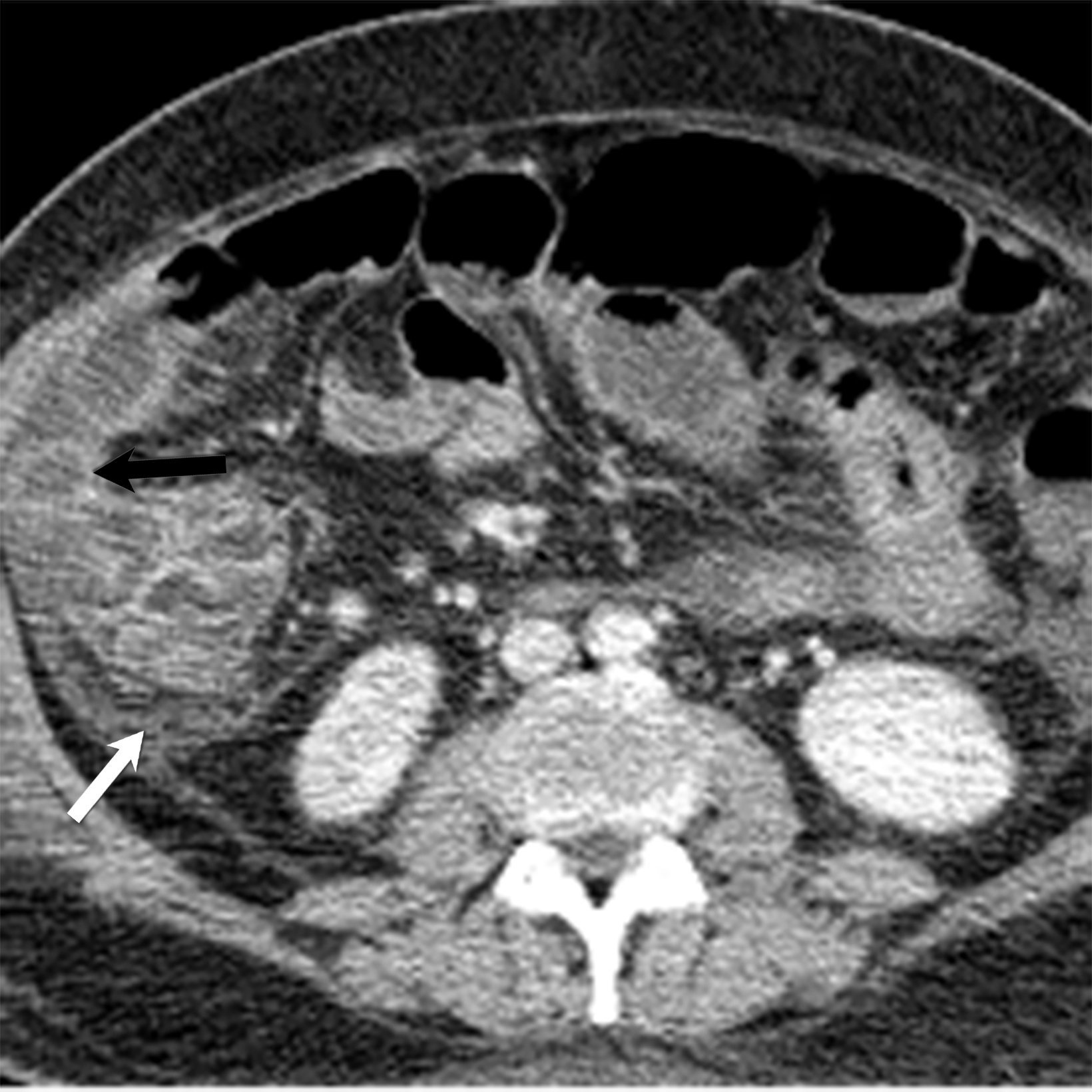
Polyarteritis nodosa (PAN) is a systemic necrotizing vasculitis that may be triggered either idiopathically or by viral infection such as hepatitis or human immunodeficiency virus (HIV). Compared with Kawasaki disease, PAN typically presents with subacute onset and is more commonly observed in the adult population, particularly among Alaskan and Kuwaiti natives.7 The diagnosis of systemic PAN is established by the presence of three of the following: significant weight loss, livedo reticularis, testicular pain, myalgia, neuropathy, hypertension, elevated renal function, hepatitis B infection, angiographic abnormalities demonstrating aneurysms or occlusions and biopsy-proven vasculitis.
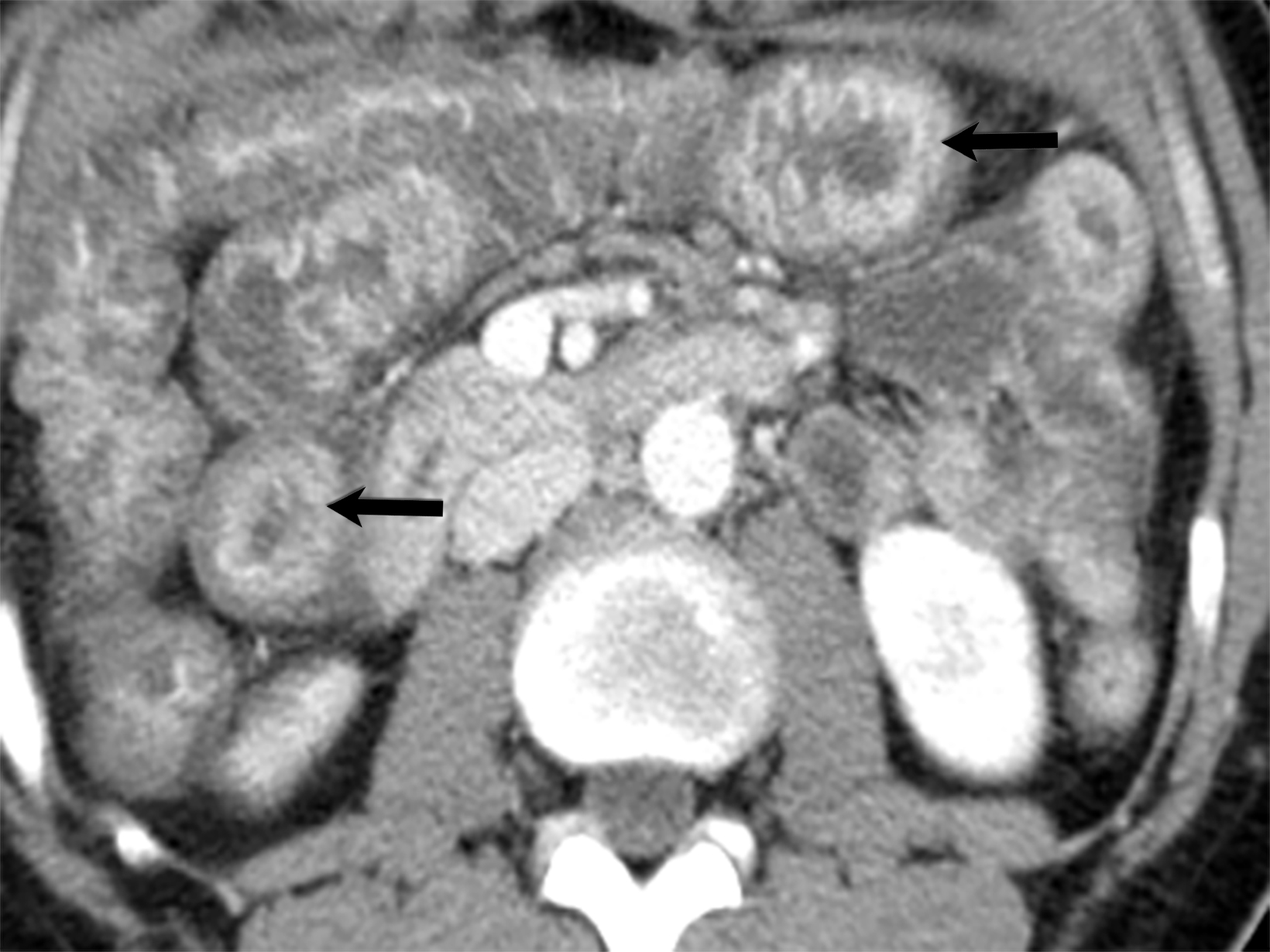
A hallmark of PAN is multiple aneurysms, most commonly in the kidneys (80-90%), followed by the gastrointestinal tract (50-70%), liver (50-60%), coronary arteries (50%), spleen (45%) and pancreas (25-35%). Computed tomography (CT) and MRI vascular findings include segmental mural thickening, submucosal edema, abnormal hyperenhancement with a striated pattern, arterial stenosis, and visceral aneurysms (Figure 5).23,24 Imaging can also reveal renal or bowel infarcts, perforation, hemorrhage, or intra-muscular hematoma.25,26 The diagnosis often relies on angiographic findings of aneurysms up to 1 cm in diameter with a pathological correlation of fibrinoid necrosis.7,25
Small-vessel Vasculitis
Granulomatosis with Polyangiitis
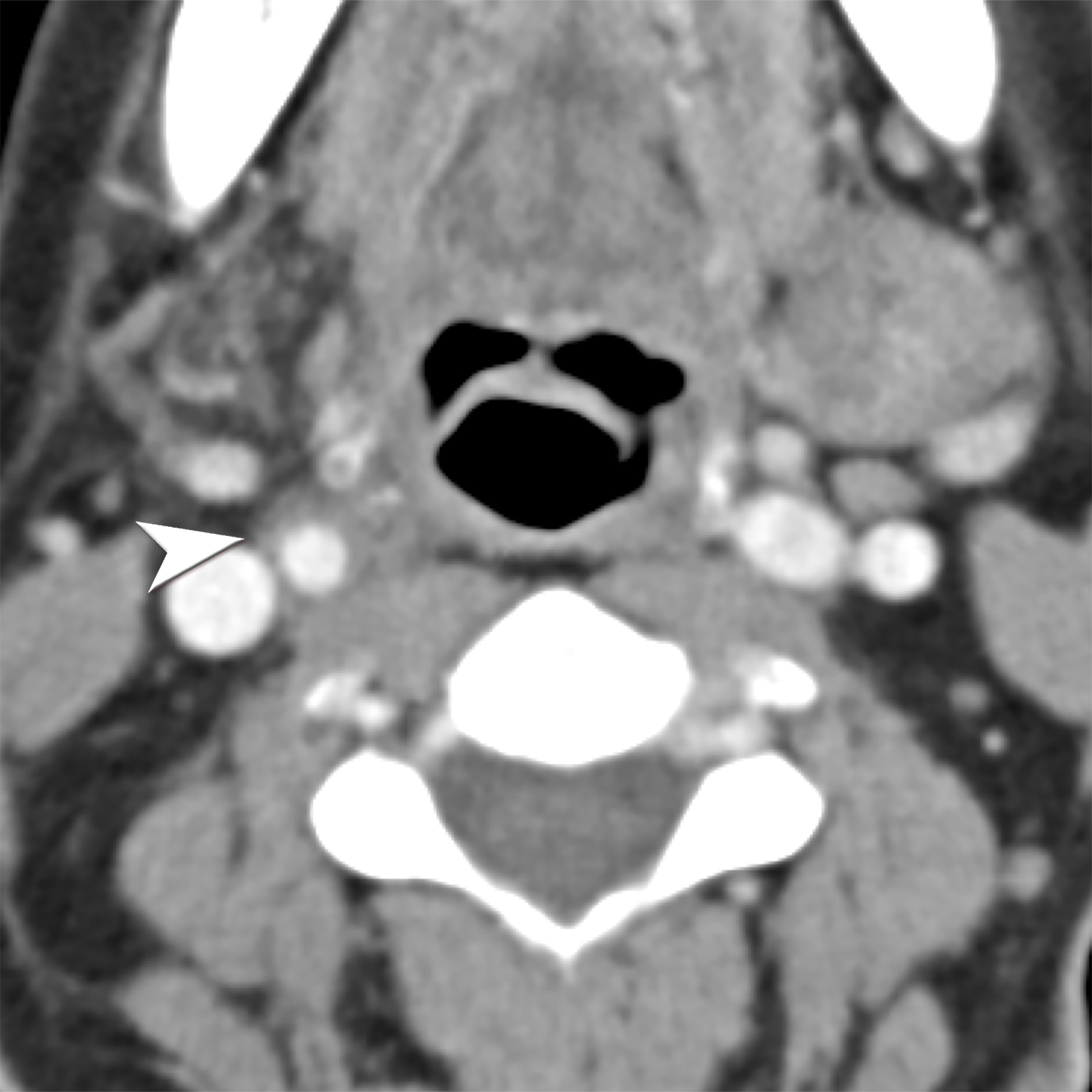
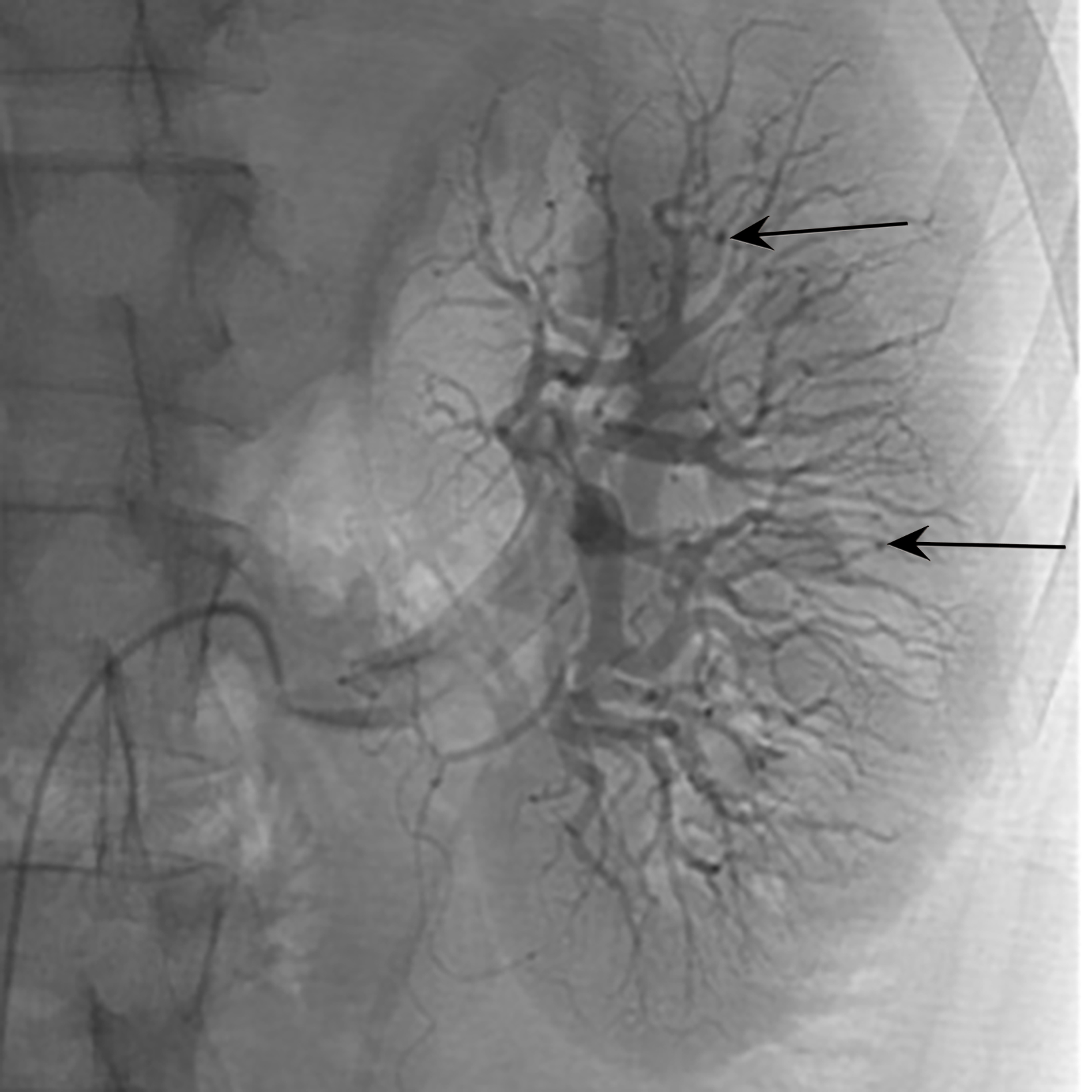
Granulomatosis with polyangiitis (GPA), formerly known as Wegner’s granulomatosis, is an angiogenic multisystem necrotizing disease characterized by a triad of upper and lower respiratory tract granulomas, vasculitis, and renal involvement (necrotizing crescentic glomerulonephritis). GPA primarily affects adults between the ages of 64 and 75, with an incidence of 3 cases per mil- lion people in the United States. Diagnosis requires at least 2 of the following: urinary sediment containing red blood cell casts, abnormal chest radiograph, presence of oral ulcers or nasal discharge, and granulomatous inflammation on biopsy.27-29
Magnetic resonance imaging reveals increased T2 signal and postcontrast enhancement in early disease and low T2 nonenhancing fibrosis in late-stage disease, which can manifest as enophthalmos and subglottic or tracheal stenosis.27,30 About 25% of GPA patients present with sinonasal disease with nonspecific maxillary mucosal thickening on CT. Late-stage disease may show osseous destruction involving the nasal septum, turbinates, and anterior ethmoid region, potentially leading to a saddle-nose deformity, a hallmark of GPA.27 Pulmonary findings in CT are nonspecific they include nodules, consolidations, and ground-glass opacities. 28 Renal involvement appears on ultrasound as enlarged echogenic kidneys in early stage — and shrunken kidneys in late — stage disease.
Meanwhile, MRI shows wedge-shaped T2 hyperintensity and hypoenhancement in nephritis or ischemia (Figure 6).23 Gastrointestinal manifestations encompass segmental hyperenhancing bowel-wall thickening with mesenteric engorgement and, less commonly, granulomatous colitis, granulomatous pancreatic mass, splenic infarct or hemorrhage, and gastritis.23
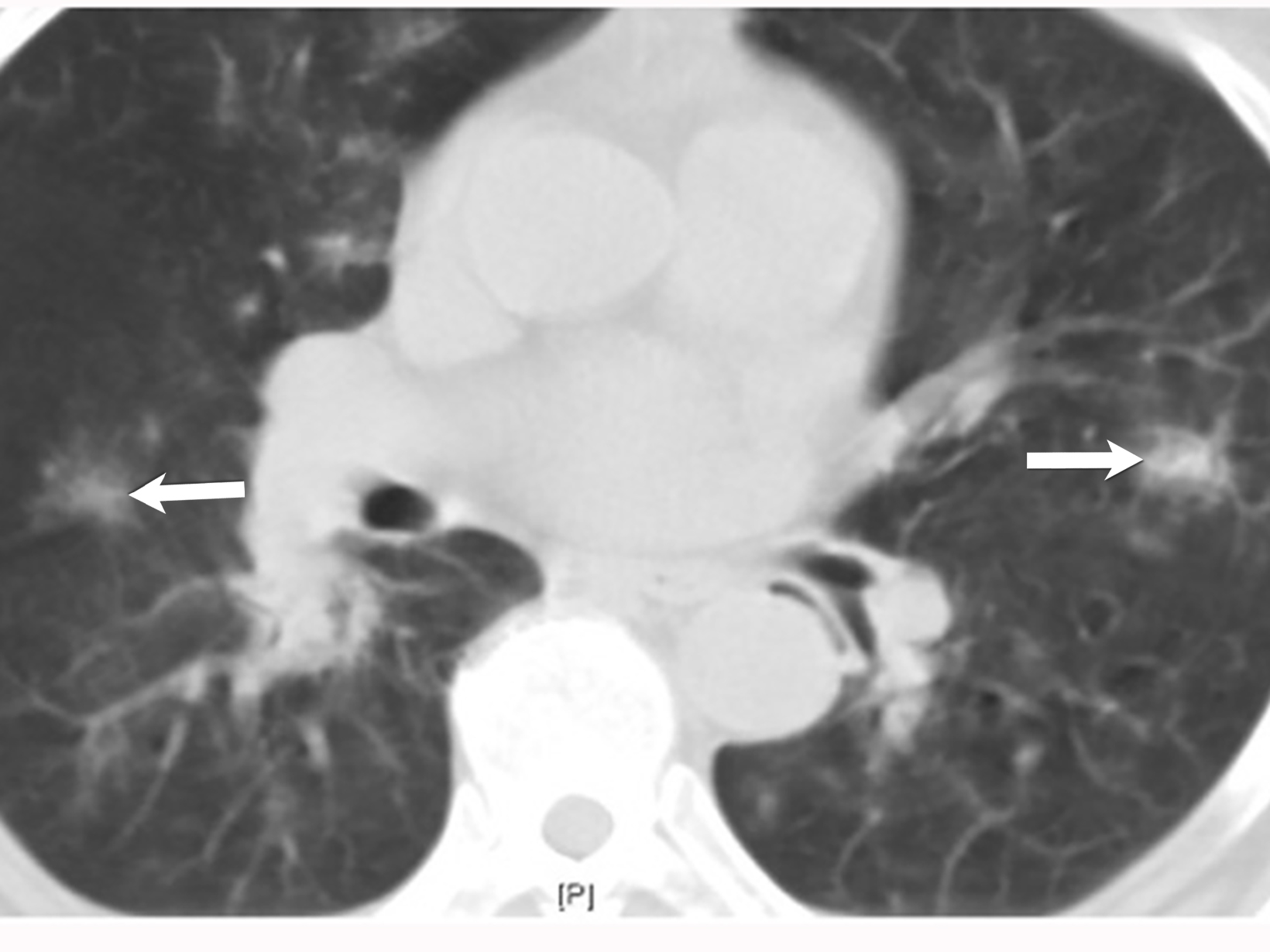
Microscopic Polyangiitis
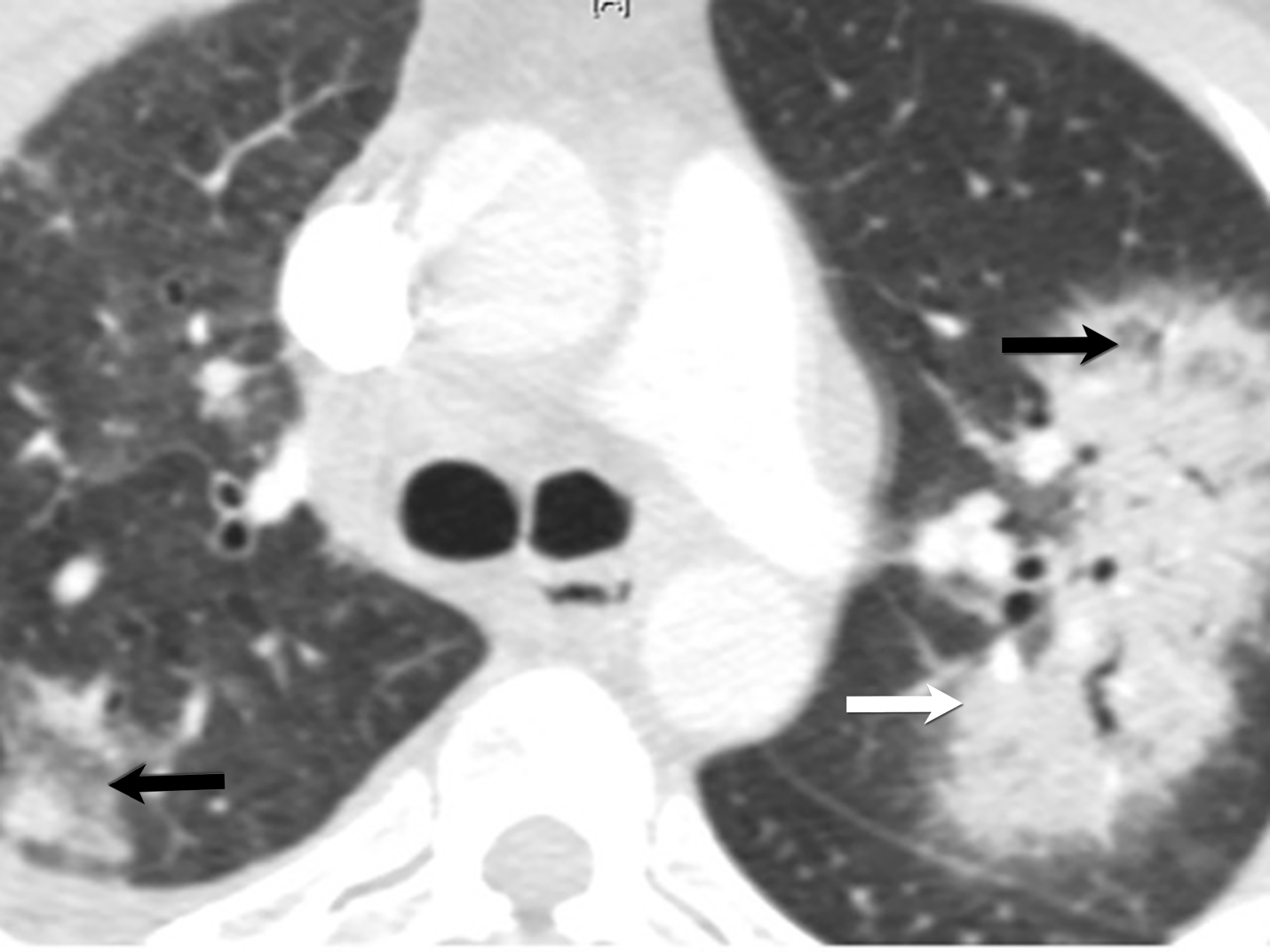
Microscopic polyangiitis (MPA) is a necrotizing vasculitide, like GPA, with predominance in Asian countries such as China and Japan.31 Clinical and imaging presentation can be variable depending on the affected organs and include kidney failure, hemoptysis, dyspnea, pleuritic pain, and constitutional symptoms (Figure 7).32 Upper airway complications are less common in MPA com- pared to GPA. 33 The 2020 ACR-EULAR Classification Criteria for small vessel vasculitis include ANCA subtype as a major criterion between GPA and MPA. 12
Patients with pulmonary symptoms suspected of having MPA should undergo chest CT, which can demonstrate nodules with or without cavitation, alveolar opacities, or pleural lesions. Non-contrast CT is the preferred imaging study, as these patients may also have renal impairment.31
Eosinophilic Granulomatosis With Polyangiitis
Eosinophilic granulomatosis with polyangiitis (EGPA), previously known as Churg-Strauss syndrome, is an eosinophil-rich multisystem necrotizing granulomatous inflammation. The typical clinical presentation typically includes asthma, eosinophilia, palpable purpura, and a positive PR3-ANCA test found in 50% of cases. EGPA commonly involves the lungs, heart, gastrointestinal tract, spleen, and kidneys (Figure 8).
Cardiac involvement carries an unfavorable prognosis and is an independent predictor of mortality. Cardiac MR can assess the extent of cardiac involvement, including cardiomyopathy (30%, with late gadolinium enhancement), pericardial effusion (22%), and valvular disease (mainly affecting the mitral valve).3,7
Henoch-Schonlein Vasculitis
Henoch-Schonlein vasculitis, also known as IgA vasculitis, is an IgA-mediated immune vasculitis that primarily affects the kidneys, gastrointestinal tract, skin, lungs, joints, and central nervous system. The condition is commonly observed in pediatric males and typically presents with either an upper respiratory or gastrointestinal infection.34 Diagnostic criteria include purpura/ petechiae with lower limb predominance and at least one of the following: arthritis, diffuse abdominal pain, renal involvement, or histopathology revealing IgA deposits or leukocytoplastic vasculitis.35
The gastrointestinal system, commonly the ileum, is involved in 60% of patients, with radiography showing smooth fold thickening, known as thumbprinting or irregular wall thickening.36 CT features are nonspecific and may include bowel-wall thickening, mural hyperdensity in noncontrast images, indicating submucosal hemorrhage, or focal mural hypoenhancement, indicating isch- emia (Figure 9). Differential diagnoses include inflammatory bowel disease, enteritis, ischemic bowel, and malignancy. Imaging also plays an essential role in diagnosing complications such as intussusception, perforation, and obstruction.23,37
Lupus Vasculitis
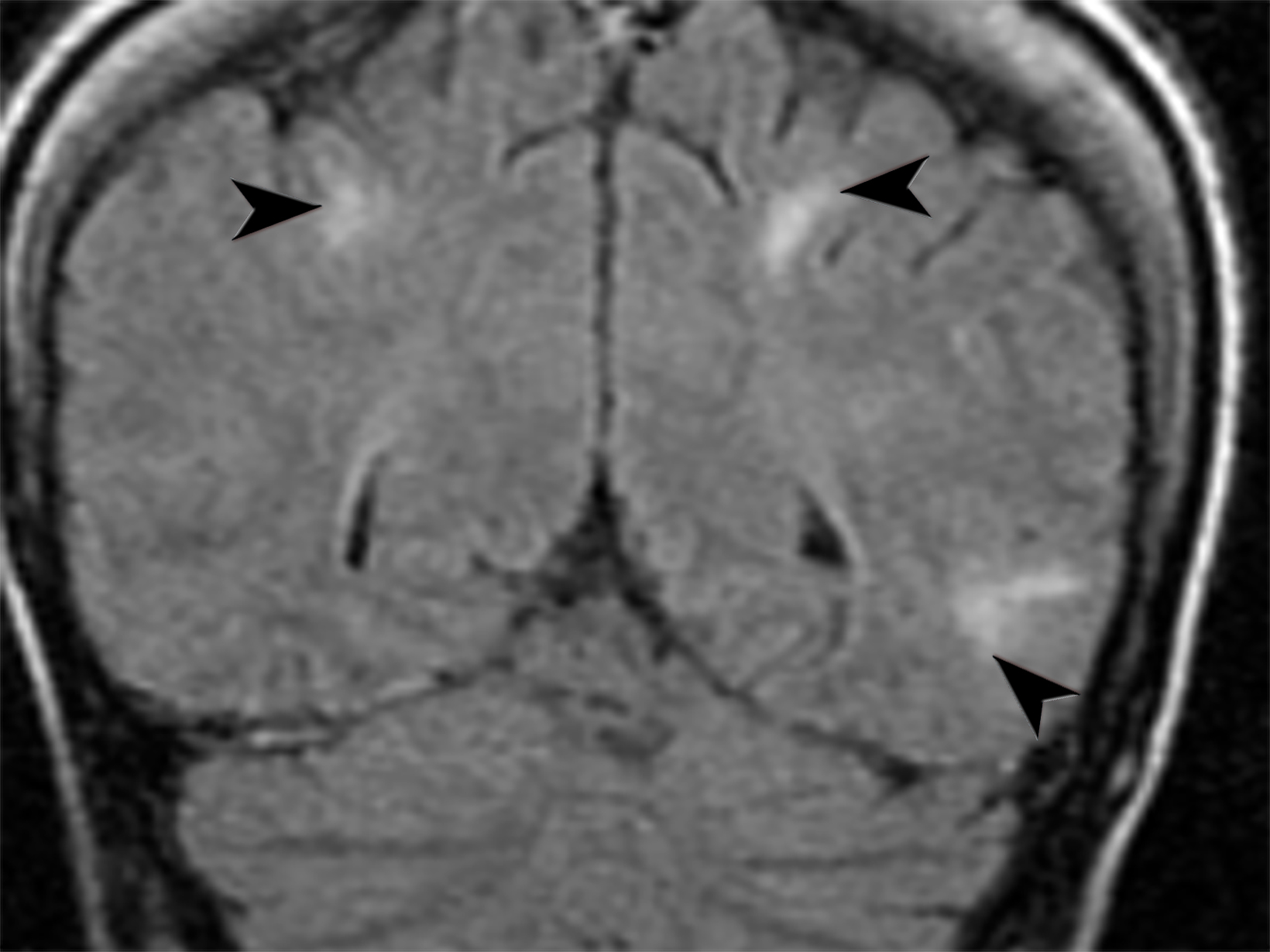
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory condition with a broad spectrum of manifestations, including vasculitis, which can lead to severe morbidity and mortality. 38 Cutaneous lupus vasculitis (19-28%) presents with palpable purpura, petechiae, splinter hemorrhages, and superficial ulcerations. 39 Neuropsychologic SLE secondary to vasculitis (31%) presents with multiple high T2-weighted/FLAIR focal lesions in the subcortical white matter and basal ganglia in MRI.40,41 Lupus enteritis (0.2-14.2%) presents with dilated bowel, focal, or diffuse bowel-wall thickening, abnormal bowel-wall enhancement, engorged mesenteric vessels, ascites, and lymphadenopathy in abdominal CT (Figure 10). Renal vasculitis coexisting with lupus nephritis increases the risk of end-stage renal disease.42 Pulmonary vasculitis often leads to diffuse alveolar hemorrhage with ground-glass opacifications and pleural effusions in chest CT.43
Conclusion
Vasculitis represents a complex spectrum of disorders characterized by inflammation of blood vessels. Given its myriad presentations in the emergencies and potential for severe complications, timely and accurate diagnosis is paramount.
Although tissue biopsy remains definitive, its limitations underscore the critical role of imaging in diagnosis within emergency settings.
Radiologists equipped with a deep understanding of vasculitis’ imaging manifestations are essential to the diagnostic and management process. Collaborative efforts, melding clinical and radiological insights, are vital for optimal outcomes for emergent cases of vasculitis.
References:
- Jatwani S, Goyal A. Vasculitis. StatPearls. 2023.
- Sreih AG, Cronin K, Shaw DG, et al. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J Rare Dis. Apr 21 2021;16(1):184. doi:10.1186/s13023-021-01794-5
- Ha HK, Lee SH, Rha SE, et al. Radiologic features of vasculitis involving the gastrointestinal tract. Radiographics. May-Jun 2000;20(3):779-94. doi:10.1148/ radiographics.20.3.g00mc02779
- Suresh E. Diagnostic approach to patients with suspected vasculitis. Postgrad Med J. Aug 2006;82(970):483-8. doi:10.1136/pgmj.2005.042648
- Bahrami M, Mohammadi H, Mirgaloyebayat H, et al. The role of 18F-fluorodeoxyglucose PET/computed tomography in the diagnosis and monitoring of large vessel vasculitides - a review article. Am J Nucl Med Mol Imaging. 2023;13(4):127-135.
- Guggenberger KV, Bley TA. Imaging in vasculitis. Curr Rheumatol Rep. Jun 19 2020;22(8):34. doi:10.1007/s11926-020-00915-6
- Broncano J, Vargas D, Bhalla S, Cummings KW, Raptis CA, Luna A. CT and MR imaging of cardiothoracic vasculitis. Radiographics. Jul-Aug 2018;38(4):997-1021. doi:10.1148/rg.2018170136
- Aghayev A. Multimodality imaging of large-vessel vasculitis, from the AJR special series on inflammation. AJR Am J Roentgenol. 2022;218(2):213-222. doi:10.2214/ajr.21.26150
- Prieto-González S, Arguis P, Cid MC. Imaging in systemic vasculitis. Curr Opin Rheumatol. Jan 2015;27(1):53-62. doi:10.1097/bor.0000000000000130
- Winkler A, True D. Giant cell arteritis: 2018 review. Mo Med. Sep-Oct 2018;115(5):468-470.
- Pepper K. Giant cell arteritis. Postgrad Med. Jan 2023;135(sup1):22-32. doi:10.1080/00325481.2023.2190288
- Ecclestone T, Watts RA. Classification and epidemiology of vasculitis: emerging concepts. Best Pract Res Clin Rheumatol. Jul 17 2023:101845. doi:10.1016/j. berh.2023.101845
- Spira D, Kötter I, Ernemann U, et al. Imaging of primary and secondary inflammatory diseases involving large and medium-sized vessels and their potential mimics: a multitechnique approach. AJR Am J Roentgenol. Mar 2010;194(3):848-56. doi:10.2214/ajr.09.3367
- Rutter M, Bowley J, Lanyon PC, Grainge MJ, Pearce FA. A systematic review and meta-analysis of the incidence rate of Takayasu arteritis. Rheumatology (Oxford). Nov 3 2021;60(11):4982-4990. doi:10.1093/rheumatology/keab406
- Serra R, Butrico L, Fugetto F, et al. Updates in pathophysiology, diagnosis and management of Takayasu Arteritis. Ann Vasc Surg. 2016/08/01/ 2016;35:210-225. doi:https://doi.org/10.1016/j.avsg.2016.02.011
- Pugh D, Karabayas M, Basu N, et al. Large-vessel vasculitis. Nat Rev Dis Primers. Jan 6 2022;7(1):93. doi:10.1038/s41572-021-00327-5
- Barra L, Kanji T, Malette J, Pagnoux C. Imaging modalities for the diagnosis and disease activity assessment of Takayasu’s arteritis: A systematic review and meta-analysis. Autoimmunity Reviews. 2018/02/01/ 2018;17(2):175-187. doi:https://doi.org/10.1016/j.autrev.2017.11.021
- Esatoglu SN, Hatemi G. Takayasu arteritis. Curr Opin Rheumatol 2022;34(1)
- Tsujioka Y, Handa A, Nishimura G, et al. Multisystem imaging manifestations of Kawasaki Disease. RadioGraphics. 2022;42(1):268-288. doi:10.1148/rg.210070
- Hoang MP, Park J. Vasculitis. Hospital-Based Dermatopathology. Feb 29 2020:245-96.
- Srinivasan R, Weller R, Chelliah A, Einstein AJ. Multimodality cardiac imaging in a patient with Kawasaki Disease and Giant Aneurysms. Case Rep Pediatr. 2016;2016:4298098. doi:10.1155/2016/4298098
- Singhal M, Singh S, Gupta P, Sharma A, Khandelwal N, Burns JC. Computed tomography coronary angiography for evaluation of children with Kawasaki Disease. Curr Probl Diagn Radiol. 2018/07/01/ 2018;47(4):238-244. doi:https://doi.org/10.1067/j.cpradiol.2017.09.013
- Amouei M, Momtazmanesh S, Kavosi H, Davarpanah AH, Shirkhoda A, Radmard AR. Imaging of intestinal vasculitis focusing on MR and CT enterography: a two-way street between radiologic findings and clinical data. Insights Imaging. Sep 4 2022;13(1):143. doi:10.1186/s13244-022-01284-7
- Venkatanarasimha N, Irani F. Polyarteritis Nodosa. Radiology. 2023/03/01 2022;306(3):e221255. doi:10.1148/radiol.221255
- Jee KN, Ha HK, Lee IJ, et al. Radiologic findings of abdominal polyarteritis nodosa. AJR Am J Roentgenol. 2000;174(6):1675-1679. doi:10.2214/ ajr.174.6.1741675
- Bixio R, Orsolini G, Fassio A, Rossini M, Viapiana O. Clinical image: ultrasound findings and magnetic resonance imaging comparison in the muscular involvement in polyarteritis nodosa. Clin Rheumatol. 2023/03/01 2023;42(3):967-969. doi:10.1007/s10067-022-06461-z
- Cleary JO, Sivarasan N, Burd C, Connor SEJ. Head and neck manifestations of granulomatosis with polyangiitis. Br J Radiol. Mar 1 2021;94(1119):20200914. doi:10.1259/bjr.20200914
- Li J, Li C, Li J. Thoracic manifestation of Wegener’s granulomatosis: computed tomography findings and analysis of misdiagnosis. Exp Ther Med. Jul 2018;16(1):413-419. doi:10.3892/etm.2018.6154
- Garlapati P, Qurie A. Granulomatosis with polyangiitis. StatPearls. 2023.
- Allen SD, Harvey CJ. Imaging of Wegener’s granulomatosis. Brit J Radiol. 2007/09/01 2007;80(957):757-765. doi:10.1259/bjr/34705892
- Kitching AR, Anders H-J, Basu N, et al. ANCA-associated vasculitis. Nat Rev Dis Primers 2020/08/27 2020;6(1):71. doi:10.1038/s41572-020-0204-y
- Sacoto G, Boukhlal S, Specks U, Flores-Suárez LF, Cornec D. Lung involvement in ANCA-associated vasculitis. La Presse Médicale. 2020/10/01/ 2020;49(3):104039. doi:https://doi.org/10.1016/j.lpm.2020.104039
- Yaseen K, Mandell BF. ANCA associated vasculitis (AAV): a review for internists. Postgrad Med J. 2023/01/02 2023;135(sup1):3-13. doi:10.1080/0032 5481.2022.2102368
- Roache-Robinson P, Hotwagner DT. Henoch-Schonlein Purpura. StatPearls. 2023.
- Reamy BV, Servey JT, Williams PM. Henoch-Schönlein purpura (IgA vasculitis): rapid evidence review. Am Fam Physician. Aug 15 2020;102(4):229-233.
- Glasier CM, Siegel MJ, McAlister WH, Shackelford GD. Henoch-Schonlein syndrome in children: gastrointestinal manifestations. AJR Am J Roentgenol.1981/06/01 1981;136(6):1081-1085. doi:10.2214/ajr.136.6.1081
- Nota ME, Gökemeijer JD, van der Laan JG. Clinical usefulness of abdominal CT-scanning in Henoch-Schönlein vasculitis. Neth J Med. Mar 1995;46(3):142-5. doi:10.1016/0300-2977(94)00059-i
- >Calle-Botero E, Abril A. Lupus Vasculitis. Curr Rheumatol Rep. 2020/08/26 2020;22(10):71. doi:10.1007/s11926-020-00937-0
- Sharma A, Dhooria A, Aggarwal A, Rathi M, Chandran V. Connective tissue disorder-associated vasculitis. Curr Rheumatol Rep. 2016/04/20 2016;18(6):31. doi:10.1007/ s11926-016-0584-x
- Cohen D, Rijnink EC, Nabuurs RJ, et al. Brain histopathology in patients with systemic lupus erythematosus: identification of lesions associated with clinical neuropsychiatric lupus syndromes and the role of complement. Rheumatology (Oxford). Jan 2017;56(1):77-86. doi:10.1093/rheumatology/kew341
- Shibuya M, Leite CDC, Lucato LT. Neuroimaging in cerebral small vessel disease: Update and new concepts. Dement Neuropsychol. Oct-Dec 2017;11(4):336-342. doi:10.1590/1980-57642016dn11-040002
- Leone P, Prete M, Malerba E, et al. Lupus Vasculitis: an overview. Biomedicines. Nov 5 2021;9(11)doi:10.3390/biomedicines9111626
- Amarnani R, Yeoh SA, Denneny EK, Wincup C. Lupus and the lungs: the assessment and management of pulmonary manifestations of systemic lupus erythematosus. Front Med (Lausanne). 2020;7:610257. doi:10.3389/fmed.2020.610257
Citation
SB E, M M, CG B, M M, M V, D K, DR G.Vasculitis in the Emergency Room: The Pivotal Role of Imaging in Diagnosis and Management. Supplement to Applied Radiology. 2023; (1):44-53.
January 25, 2024