Vascular anomalies: Description, classification and nomenclature
Images
This article is accredited for one SA-CME credit. Visit appliedradiology.org/SAM2 for full SA-CME information.
“Nomenclature has been the major obstacle to our understanding and management of vascular anomalies.”1 These words were written by John Mulliken over 30 years after publishing his seminal paper on the biologic basis of vascular anomalies in 1982. The intervening decades have brought tremendous progress in classification, diagnosis and therapy of this diverse group of lesions. Still, much confusion exists in the medical community, in no small part because of inaccurate and inconsistent use of nomenclature both in the literature and in clinical practice.2
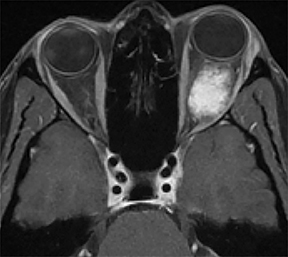
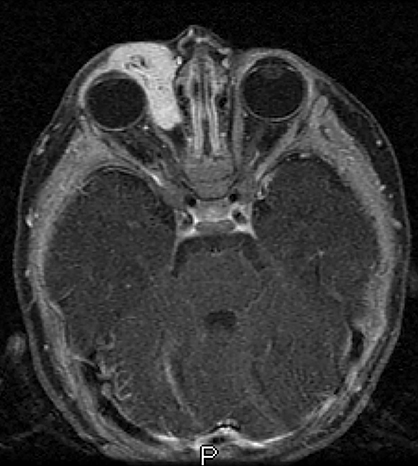
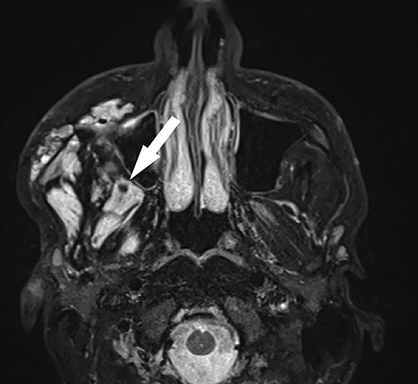
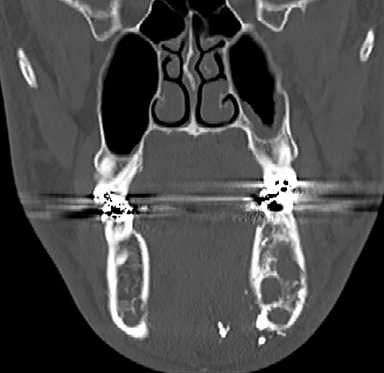
A prime example is the widespread continued use of the term “hemangioma” to refer to any vascular lesion, with little regard to actual histologic composition. Lesions inaccurately called “hemangioma” occur throughout the head and neck, including “cavernous hemangioma” described in the orbit and parapharyngeal space, and “ossifying hemangioma” of the facial nerve and facial skeleton (Figure 1). The majority of lesions incorrectly termed “hemangioma” represent venous malformations, including those listed above.3 Unfortunately, any attempt to interrogate the literature regarding these entities will yield a mixed bag of terminology that limits our ability to effectively utilize the body of published data on the topic. Following a PubMed search, Hassanein and colleagues reviewed the literature where the word “hemangioma” appeared in the title or abstract, and found it incorrectly used about 70% of the time. Most concerning was the fact that patients whose lesions were mislabeled were considerably more likely to receive inappropriate treatment (20%) than those in whom the lesion was correctly classified (0%).2
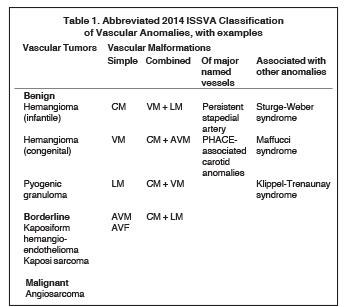
The multidisciplinary International Society for the Study of Vascular Anomalies (ISSVA) was formed in 1992 to promote research in the field of vascular anomalies and to create a uniform nomenclature that would facilitate research and clinical practice. ISSVA created a comprehensive classification scheme based on Mulliken’s work on the biologic basis of disease, updated most recently in 2014 and available at issva.org/classification. The ISSVA classification and its associated nomenclature are widely accepted as the gold standard by the numerous medical specialties involved in clinical care and research related to vascular anomalies. Table 1 presents an abbreviated and simplified version of the 2014 ISSVA classification that includes the most common lesions encountered in the head and neck.
Biologic basis of disease
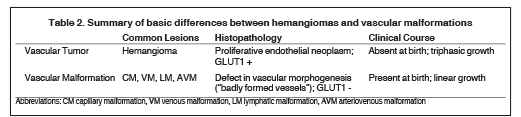
Based on cellular analysis, Mulliken and Glowacki demonstrated that the broad spectrum of vascular anomalies consisted of two major categories: vascular tumors and vascular malformations.4 The infantile hemangioma is by far the most common vascular tumor, with an incidence estimated at 4-10% of all infants and children.5 Vascular tumors are true proliferative neoplasms and demonstrate growth by endothelial hyperplasia with elevated serum markers of cellular proliferation such as VEGF and cell nuclear antigen, as well as the immunohistochemical marker glucose transporter protein-1(GLUT1). These markers are absent in vascular malformations, which are considered localized static defects of vascular morphogenesis that demonstrate a quiescent endothelium. Mulliken observed that the literal translation of “vascular malformation” is “badly formed vessels”, and that is a very apt and useful description.6 Vascular malformations are a more heterogeneous group of lesions that consist of capillary, venous, arterial and lymphatic elements that may also occur in combination.
Though clinical presentations necessarily differ based on the specific type of vascular anomaly and anatomic site of disease, there are broad clinical features that distinguish the two groups. Vascular malformations are present and fully formed at birth, and grow proportionately with the somatic growth of the child. Hemangiomas, however, are classically not present at birth but rather appear weeks to several months later. Additionally, hemangiomas demonstrate a characteristic triphasic growth pattern, with initial rapid enlargement (Phase 1 or Proliferating Phase), followed by stabilization and regression (Phase 2 or Involuting Phase), ending with complete involution (Phase 3 or Involuted Phase). The basic histologic and clinical differences between vascular tumors and malformations are summarized in Table 2.
ISSVA classification
The formation of ISSVA in 1992 formalized a biennial international workshop on vascular anomalies started by Drs. Mulliken and Andrew Young in 1976. This multidisciplinary team of international experts has continued to expand our understanding of vascular anomalies and to refine their classification. The most recent classification scheme of 2014 continues to divide vascular anomalies into vascular tumors and vascular malformations.
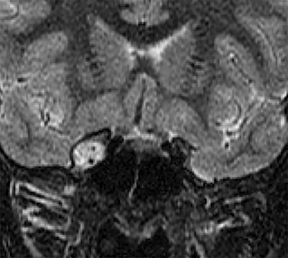
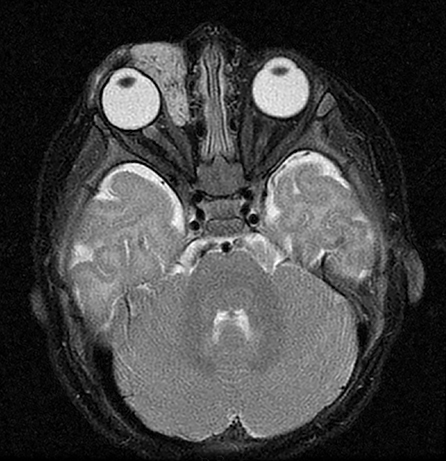
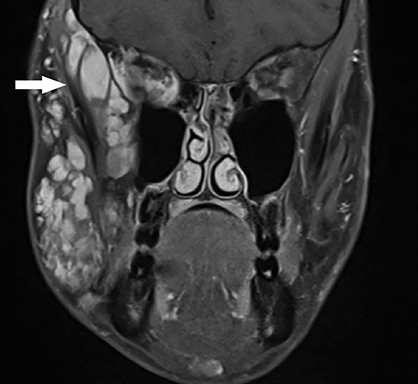
Vascular tumors are further classified as benign, locally aggressive or malignant. Benign infantile hemangiomas represent the vast majority of vascular tumors and occur most commonly in their sporadic form. They typically involve ectodermal structures such as the skin, subcutaneous soft tissues and parotid gland, but may also occur within deep tissues7 (Figure 2). Most hemangiomas do not require intervention, with the exception of lesions in which there is substantial functional or cosmetic disturbance. For example: In the head and neck, orbital involvement may necessitate therapy because of visual disturbance and the potential for the development of amblyopia.8 When a facial hemangioma occurs in a segmental distribution (typically within the midline frontonasal region or the dermatome of a trigeminal nerve branch), work-up to assess for potential underlying PHACE (posterior fossa malformations, hemangiomas, arterial anomalies, cardiovascular anomalies, eye anomalies) syndrome should be undertaken.9
Congenital hemangiomas are rare benign vascular tumors with clinical and histologic features that differ from infantile hemangiomas; they are present at birth, do not manifest the classic triphasic growth pattern associated with infantile hemangiomas and are GLUT1 negative.10 Congenital hemangiomas occur in rapidly involuting (RICH), partially involuting (PICH) or non-involuting (NICH) variants. Other rare benign vascular tumors include the pyogenic granuloma (also called lobular capillary hemangioma), which is one of the reactive vascular proliferative lesions grouped with vascular tumors.
In the borderline category, kaposiform hemangioendothelioma is a childhood tumor that may be associated with thrombocytopenia and consumptive coagulopathy, whereas Kaposi sarcoma is most commonly encountered in the immunocompromised adult. Angiosarcoma is a rare malignant vascular tumor of older adults that most often involves the scalp and skin and is associated with high rates of local recurrence and distant metastases.11
Vascular malformations are classified by vessel of origin, with further refinement based on whether they represent a malformation of a single vessel type (“simple”) or reflect a combination of vessel types (“combined”). Vascular malformations are additionally categorized by whether they involve a major named vessel or are part of a syndrome with other associated anomalies. Those lesions with arterial components are qualified as “high flow.” There are 5 types of simple vascular malformations:
• Capillary malformations (CMs): Commonly encountered capillary malformations include the cutaneous or mucosal CMs, widely termed “port-wine stains”. These may occur as part of the Sturge-Weber syndrome, where they are accompanied by ocular and intracranial vascular abnormalities.12 Unlike most vascular malformations, the nevus simplex, commonly known as “stork bite” or “angel’s kiss,” will often lighten with time and disappear during early childhood. Imaging is not typically performed for primary assessment of CMs, but rather to look for associated syndromic anomalies.
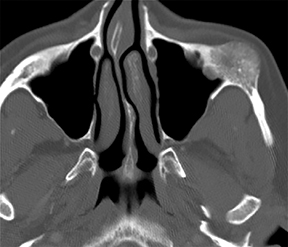
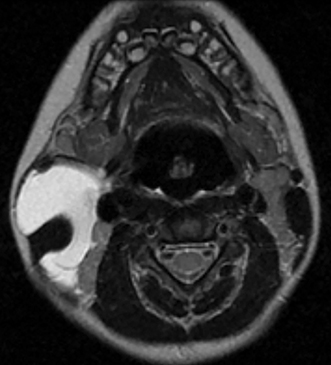
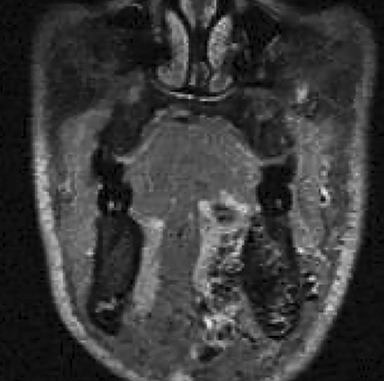
• Lymphatic malformations (LMs): These lesions are composed of dilated lymphatic channels and cysts and are further categorized as macrocystic, microcystic or mixed (Figure 3). The archaic term “cystic hygroma” generally referred to the macrocystic variety, which appears as a fluid-filled thin-walled structure with no more than a few compartments. The microcystic variety is composed of innumerable small components with thick intervening septations. Microcystic LMs infiltrate tissues diffusely and typically cannot be successfully treated with sclerotherapy.
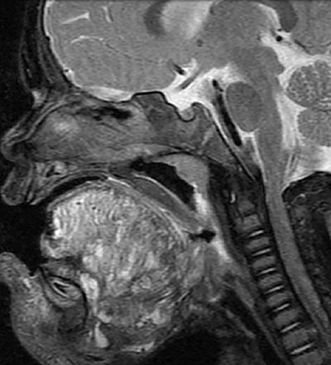
• Venous malformations (VMs): These compressible lesions are composed of malformed venous channels with sluggish internal blood flow and are often associated with focal areas of thrombosis that result in the characteristic phleboliths. Head and neck VMs involve both superficial and deep tissues and are often trans-spatial, with a predilection for the muscles of mastication (Figure 4). In blue rubber bleb syndrome, multiple cutaneous VMs are present in association with soft tissue and gastrointestinal lesions, the latter responsible for chronic anemia.13 The orbital VM incorrectly termed “cavernous hemangioma” is the most common orbital mass in adults.14
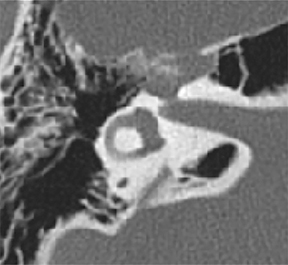
• Arteriovenous malformations (AVMs): Though these are composed of arteries, veins and intervening capillaries they are considered a single disease entity and are classified as simple malformations. These lesions may be very aggressive, with a tendency to infiltrate tissues diffusely and to involve the underlying bone, resulting in both lysis and overgrowth (Figure 5).
• Arteriovenous fistulas (AVFs): AVFs, with direct fistulous communication between arteries and veins, are much more commonly acquired and related to trauma. Congenital AVFs, like AVMs, are typically sporadic but may also occur as part of a syndrome, such as hereditary hemorrhagic telangiectasia (HHT or Osler-Weber-Rendu) syndrome.15
• Combined malformations are composed of two or more types of vascular malformations (CM, LM, VM, AVM) found within one lesion. A common combined malformation is the LM + VM (also called LVM or “venolymphatic malformation”). In addition, cutaneous CMs may be present overlying a one or more deeper simple vascular malformation (e.g. CM + AVM, CM + LM + VM).
Vascular malformations of major named vessels include anomalies affecting the origin, course, number, length or diameter of larger lymphatic, venous or arterial vessels, as well as persistent embryonal vessels. The carotid artery anomalies associated with PHACE syndrome (including aplasia/hypoplasia, stenosis and redundancy) as well as persistent stapedial arteries encountered in the temporal bone are included within this category.16
Finally, there is a long and growing list of vascular malformations associated with other anomalies. In addition to Sturge-Weber syndrome, this category includes such rare entities as Klippel-Trenaunay syndrome (CM + VM +/- LM + limb overgrowth), Proteus syndrome (CM, VM and/or LM + asymmetric somatic overgrowth) and Maffucci syndrome (VM +/- spindle cell hemangioma + enchondroma).5
Imaging of vascular anomalies
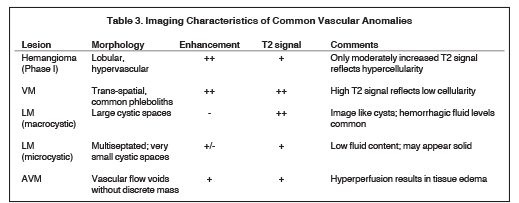
The imaging features of vascular lesions generally reflect their underlying histologic composition.6 Specifically, the MRI signal characteristics suggest both the cellular nature of hemangiomas and the vessels of origin of vascular malformations. Imaging characteristics of commonly encountered lesions are summarized in Table 3.
Old and new nomenclature
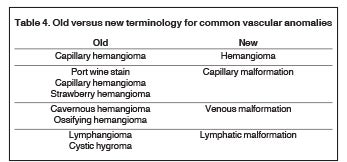
It is hoped that the utilization of archaic and inaccurate terminology will decline with improved understanding of the fundamental differences between the various vascular anomalies and with more widespread familiarity with the ISSVA classification scheme. In addition to the stubborn persistence of the term “cavernous hemangioma” throughout the literature and within everyday practice, other terms such as “port wine stain” and “lymphangioma” remain pervasive. The suffix “oma” is typically associated with tumors, and is appropriately reserved for the vascular tumors such as hemangioma and hemangioendothelioma. A summary of the modifications in terminology for some of the most common lesions is presented in Table 4.
Conclusion
The umbrella term “vascular anomalies” describes a highly diverse group of diseases ranging from the commonly encountered to the exotic that are nonetheless unified by common underlying histologic patterns. The classification scheme in widespread use today was updated in 2014 by ISSVA, and is based on John Mulliken’s critical work clarifying the biologic basis of these diseases and broadly dividing them into vascular tumors and vascular malformations. Consistent utilization of the ISSVA classification and associated terminology will help improve clinical care as well as the quality of the literature on this topic. Importantly, the term “hemangioma” should be reserved for the common benign vascular tumors of infancy. The frequently utilized term “cavernous hemangioma” is a misnomer referring to a venous malformation. The full ISSVA classification is available at issva.org/classification.
References
- Mulliken JB. Classification of Vascular Anomalies. In: Mulliken JB, Burrow PE, Fishman SJ, eds. Mulliken & Young’s Vascular Anomalies Hemangiomas and Malformations, 2nd Edition. Oxford: Oxford University Press; 2013:22.
- Hassanein AH, Mulliken JB, Fishman SJ, et al. Evaluation of terminology for vascular anomalies in current literature. Plast Reconstr Surg. 2011; 127(1):347–351.
- Greene AK, Rogers GF, Mulliken JB. Intraosseous “hemangiomas” are malformations and not tumors. Plast Reconstr Surg. 2000; 119(6):1949-1950; author reply 1950.
- Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconstr Surg. 1982; 69(3):412-422.
- Wassef M, Blei F, Adams D, et al. Vascular anomalies classification: recommendations from the International Society for the Study of Vascular Anomalies. Pediatrics. 2015; 136(1):e203-214. doi:10.1542/peds.2014-3673.
- Griauzde J, Srinivasan A. Imaging of vascular lesions of the head and neck. Radiol Clin North Am. 2015; 53(1):197-213. doi: 10.1016/j.rcl.2014.09.001.
- Maguiness SM, Frieden IJ. Management of difficult infantile haemangiomas. Arch Dis Child. 2012; 97(3):266-271. doi: 10.1136/archdischild-2011-300851.
- Spence-Shishido AA, Good WV, Baselga E,. Hemangiomas and the eye. Clin Dermatol. 2015; 33(2):170-182. doi:10.1016/j.clindermatol.2014.10.009.
- Garzon MC, Epstein LG, Heyer GL, et al. PHACE Syndrome: Consensus-derived diagnosis and care recommendations. J Pediatr. 2016;178:24-33.e2. doi: 10.1016/j.jpeds.2016.07.054.
- Liang MG, Frieden IJ. Infantile and congenital hemangiomas. Semin PediatrSurg. 2014; 23(4):162-167. doi:10.1053/j.
- Mullins B, Hackman T. Angiosarcoma of the head and neck. Int Arch Otorhinolaryngol. 2015; 19(3):191–195.
- Sudarsanam A, Ardern-Holmes SL. Sturge-Weber syndrome: from the past to the present. Eur J Paediatr Neurol. 2014; 18(3):257-266. doi:10.1016/j.ejpn.2013.10.003.
- Thrash B, Patel M, Shah KR, et al. Cutaneous manifestations of gastrointestinal disease: part II. J Am Acad Dermatol. 2013; 68(2):211.e1-33; quiz 244-246. doi: 10.1016/j.jaad.2012.10.036.
- Shields JA, Shields CL, Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004; 111(5):997–1008.
- Sautter NB, Smith TL. Treatment of hereditary hemorrhagic telangiectasia-related epistaxis. Otolaryngol Clin North Am. 2016;49(3):639-654. doi: 10.1016/j.otc.2016.02.010.
- Oza VS, Wang E, Berenstein A, et al. PHACES association: a neuroradiologic review of 17 patients. AJNR Am J Neuroradiol. 2008; 29(4):807-813. doi: 10.3174/ajnr.A0937.
Citation
DR S.Vascular anomalies: Description, classification and nomenclature. Appl Radiol. 2018; (9):8-13.
September 22, 2018