Two RadNet Subsidiaries Receive FDA Clearance for AI Algorithms
 RadNet, Inc. has announced US FDA clearance for its subsidiaries DeepHealth Saige-DX mammography and Quantib Prostate 2.0 MRI artificial intelligence (AI) algorithms.
RadNet, Inc. has announced US FDA clearance for its subsidiaries DeepHealth Saige-DX mammography and Quantib Prostate 2.0 MRI artificial intelligence (AI) algorithms.
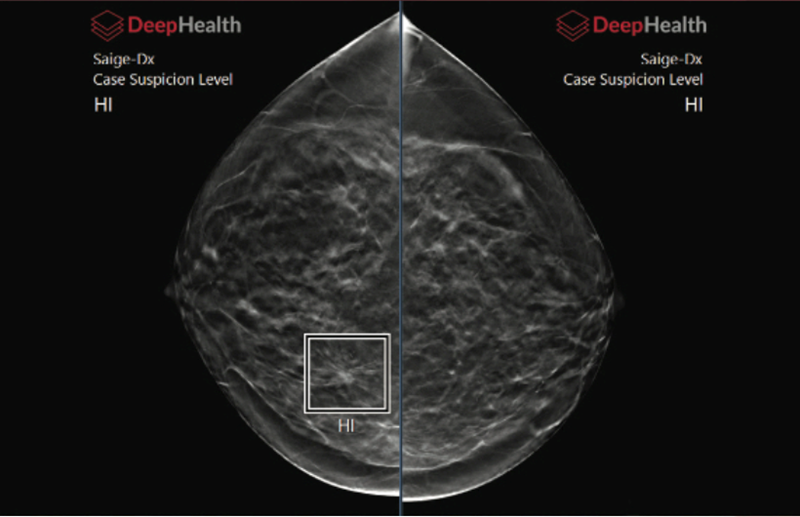
Saige-Dx is a cancer detection tool that enables radiologists to more effectively detect the presence or absence of breast cancer with the use of artificial intelligence. DeepHealth's powerful new AI technology automatically identifies suspicious lesions in mammograms and assigns a suspicion level to each finding and to the entire case. It helps detect and diagnose breast cancer earlier while reducing unnecessary recalls.
"In our study to support FDA clearance, all 18 radiologists who participated exhibited improved performance when using Saige-Dx, resulting in the largest increase in performance across all mammography AI products on the market to date. The improved performance was reflected in both an increase in the percentage of the cancers detected and a lower false positive rate when using Saige-Dx," said Bill Lotter, Ph.D., CTO, and co-founder of DeepHealth.
"Saige-Dx is built on our advanced deep-learning algorithms. Our highly accurate AI, as is described in a Nature Medicine article last year, showed the ability to find cancer one to two years earlier than fellowship-trained breast imaging radiologists. The approval of Saige-Dx represents another step forward in advancing cancer care. The feedback from physicians who have worked with our software tools is overwhelmingly positive, making them more accurate and efficient at interpreting mammography images," said Gregory Sorensen, MD, CEO, and co-founder of DeepHealth.
Quantib Prostate is an AI-based software solution that advances the MRI prostate reporting workflow and is accessible directly from the radiologist's reading station. The solution comes with a suite of tools to improve reporting quality and speed, including AI-based segmentations and volumetry, PSA density calculation, precise registration and movement correction, one-click segmentation of lesion candidates, PI-RADS scoring support, and standardized reporting to facilitate easy and comprehensive communication of results. FDA 510k special clearance has been given for a major upgrade to the solution (from release 1.3) that now includes fully automatic prostate zone segmentation (in addition to prostate gland segmentation) and automated initiation of localization of lesions on the PI-RADS sector map.
"We are delighted to present this update of Quantib Prostate, including a selection of new features that our customers requested over the past few months. We seek to continuously improve our software to support radiologists and urologists in the best way possible, and we deliver updated solutions to our customers as quickly as possible," said Arthur Post Uiterweer, CEO of Quantib.
Howard Berger, MD, President and Chief Executive Officer of RadNet, said, "We are very proud that two of our subsidiaries, DeepHealth and Quantib, have received FDA clearance for their flagship products. Artificial intelligence will have a transforming impact on radiology and cancer care, and we are committed to delivering those advances to patients and healthcare providers. These recent approvals will help us drive improved patient outcomes while lowering costs. The Biden administration, through its Cancer Moonshot program, is committed to significantly reducing the mortality rates from cancer in the coming decades. We believe that these AI tools will play an important role in the early detection and diagnosis of cancer, resulting in improved survival rates and better patient outcomes."