Somatostatin Receptor PET Imaging of Physiologic and Benign Processes: Implications for Image Interpretation, Avoiding Pitfalls, and Clinical Applications
Images
Abstract
Purpose: Somatostatin receptors (SSTR) are expressed by neuroendocrine cells in various organs, including in the spleen, thyroid gland, pituitary gland, and adrenal glands. Neuroendocrine tumors (NETs), which often demonstrate high levels of SSTR2, can be detected by positron emission tomography (PET) imaging with somatostatin analogs labeled with either 68Ga or 64Cu. The most common of these analogues (DOTATATE, DOTATOC, and DOTANOC) bind to SSTR2, with affinity to additional SSTR isoforms variable among the three tracers. Tracer activity due to benign processes and variations in normal tissue SSTR expression has implications for both the interpretation of SSTR PET imaging as well as potential future applications of this modality.
Methods: PubMed was searched separately using “DOTATATE,” “DOTATOC,” and “DOTANOC” as keywords, and results pertaining to neoplasms such as NET, pheochromocytoma, paraganglioma, and meningio- ma were excluded.
Results: Relevant original articles, case reports, and review articles were grouped between physiologic and benign observations regarding SSTR PET. A comprehensive review of the literature provided insights into image interpretation and new potential applications in assessing inflammatory disorders.
Conclusions: Variations in splenic and pancreatic tissue can cause diagnostic uncertainty and potential misinterpretation. Preliminary data suggest a role for SSTR PET to characterize atherosclerosis and sarcoidosis.
Keywords: Gallium-68, Copper-64, Biodistribution, Uncinate process, Splenule
Introduction
A neuropeptide first described in 1973,1 somatostatin generally serves to inhibit the release of other hormones such as growth hormone, insulin, and prolactin in addition to its role as a neurotransmitter.2 Somatostatin exerts its effects by binding to somatostatin receptors (SSTR), which are expressed on the surface of neuroendocrine cells. The five isoforms of SSTR, identified as SSTR1-5, have different patterns of expression depending on anatomic location. For example, SSTR2 is highly expressed in the spleen,3 SSTR1 and SSTR2 are expressed in the thyroid gland,4 while varying levels of SSTR1-5 are expressed by the pancreas.5
Because most neuroendocrine tumors (NETs) contain high levels of SSTR2, scintigraphy with the somatostatin analogue 111In-DTPA-octreotide, which binds to SSTR2 with high affinity as well as to SSTR3 and SSTR5 to a lesser degree,6 has been successfully employed for imaging of NETs since the first clinical trials in 1992.7 Recently, positron-emitting SSTR radiotracers labeled with 68Ga or 64Cu and imaged with positron emission tomography/computed tomography (PET/CT) were able to achieve superior image quality with lower patient radiation exposure given their higher affinity to SSTR2 and shorter radionuclide half-lives compared to 111In-DTPA-octreotide.8 Of the PET tracers commonly used, DOTATATE binds to SSTR2 with high specificity; DOTATOC, SSTR2 and SSTR5; DOTANOC, SSTR2, SSTR3, and SSTR5.9 When labeled with the beta-emitting 177Lu, DOTATATE can act as a theranostic agent for the treatment of well-differentiated NETs by exposing tumor cells to reactive oxygen species, resulting in oxidative damage and cell death.10
Given the variable expression of SSTR in normal tissues, physiologic uptake can occasionally mimic pathology. Alternatively, SSTR activity associated with benign disorders could lead to new indications for SSTR PET imaging in patients without known or suspected NET.
In this article, we review the sources of potential false positive findings and discuss new potential non-neoplastic indications for PET/CT imaging with radiolabeled DOTATATE, DOTATOC, and DOTANOC.
Methods
A literature search of publications between January 1, 1980, and August 31, 2023, was performed using PubMed. The SSTR PET tracers in clinical use “DOTATATE,” “DOTATOC,” and “DOTANOC” were used separately as keywords, and results pertaining to neoplastic etiologies, including NET, pheochromocytoma, paraganglioma, and meningioma, were excluded. References contained within the selected articles also received consideration. No restrictions were applied regarding sample size, study design, or outcome measures. Editorials, commentaries, and letters were excluded, as well as pre-clinical studies and those which fell outside of the scope defined by physiologic and benign causes of increased SSTR activity.
Finally, a comprehensive review was created, focusing on false-positive considerations during image interpretation and the potential for imaging of inflammation with SSTR PET.
Results
Physiologic Uptake and Pitfalls
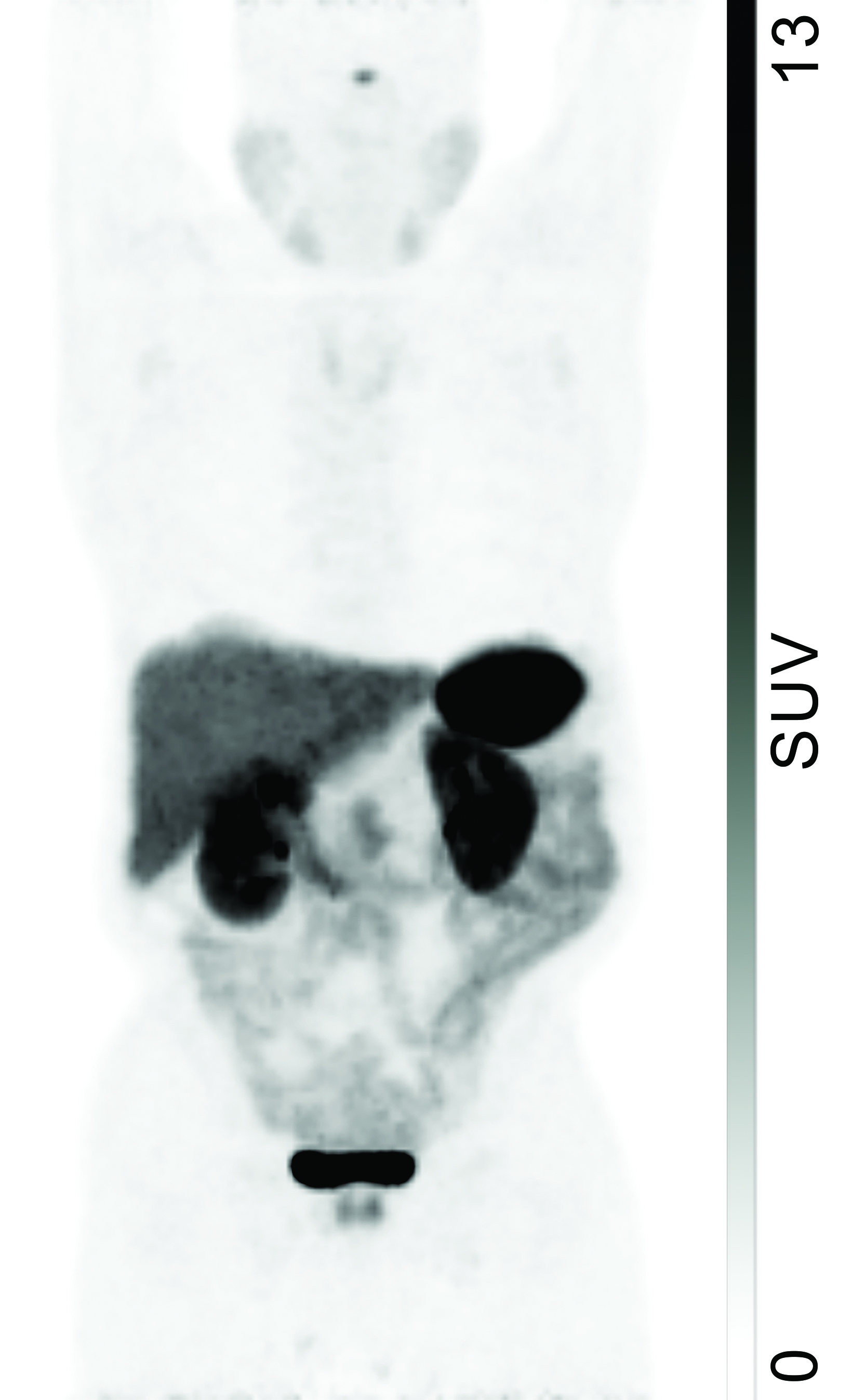
The highest physiologic uptake of 68Ga-DOTATATE is generally in the spleen (Figure 1), where increased SSTR2 expression has been found specifically in the red pulp.11 Uptake levels in the adrenal glands, pituitary gland, and kidneys demonstrate high intensity, with activity in the kidneys and urinary tract being nonspecific for SSTR expression given the presence of renal excretion.
68Ga-DOTATATE localization to the salivary glands, thyroid, and liver is moderate, with nonspecificity of hepatic activity similarly due to excretion.12 Similar patterns of intense splenic activity followed by prominent renal and hepatic activity are present in PET/CT images obtained with 68Ga-DOTATOC and 68Ga-DOTANOC.13,14 Although labeling SSTR tracers with either 68Ga or 64Cu does not affect their biodistribution, the improvement in spatial resolution resulting from the lower positron range of 64Cu suggests that 64Cu-labeled agents may achieve wider clinical use in the future.15,16
In a study of 120 patients by Boy et al, the authors observed significantly higher 68Ga-DOTATOC uptake in the uncinate process of the pancreas compared to that in the pancreatic head, body, and tail.4
This common finding among all SSTR PET agents has been described as a classic pitfall,17 which is often more diffuse and less intense compared to the well-defined focal uptake associated with NETs in this region (Figure 2).18 Lakhotia et al considered 775 patients imaged with 111In-DTPA-octreotide, 68Ga-DOTATATE, 68Ga-DOTATOC, and 68Ga-DOTANOC from eight studies and found increased physiologic uptake in the pancreatic head and uncinate process in 229 patients (29.5%).18-26 Similarly, Tabacchi et al found increased 68Ga-DOTANOC uptake at the uncinate process in 77 out of 172 patients considered (44.8%).17 The uncinate process in particular has been found to contain an increased numbers of pancreatic polypeptide cells, which highly express SSTR.27 Brabander et al observed that increased 111In-DTPA-octreotide localization to the uncinate process was more common in patients with diabetes mellitus, which is associated with increased serum pancreatic polypeptide, further supporting pancreatic polypeptide cell hyperplasia as a possible mechanism to explain uncinate process activity.20 Although Kroiss et al report successfully using standardized uptake values (SUVs) to differentiate physiologic and pathologic uptake,28 Krascz found that even in patients with suspiciously high SUVs in the uncinate process, correlative imaging with CT and MRI often reveals no underlying lesion to suggest presence of tumor.22 Thus, cautious image interpretation taking into account this potential pitfall and consideration of additional evaluation with contrast-enhanced CT or MRI are necessary for accurate interpretation of pancreatic uptake in SSTR PET.
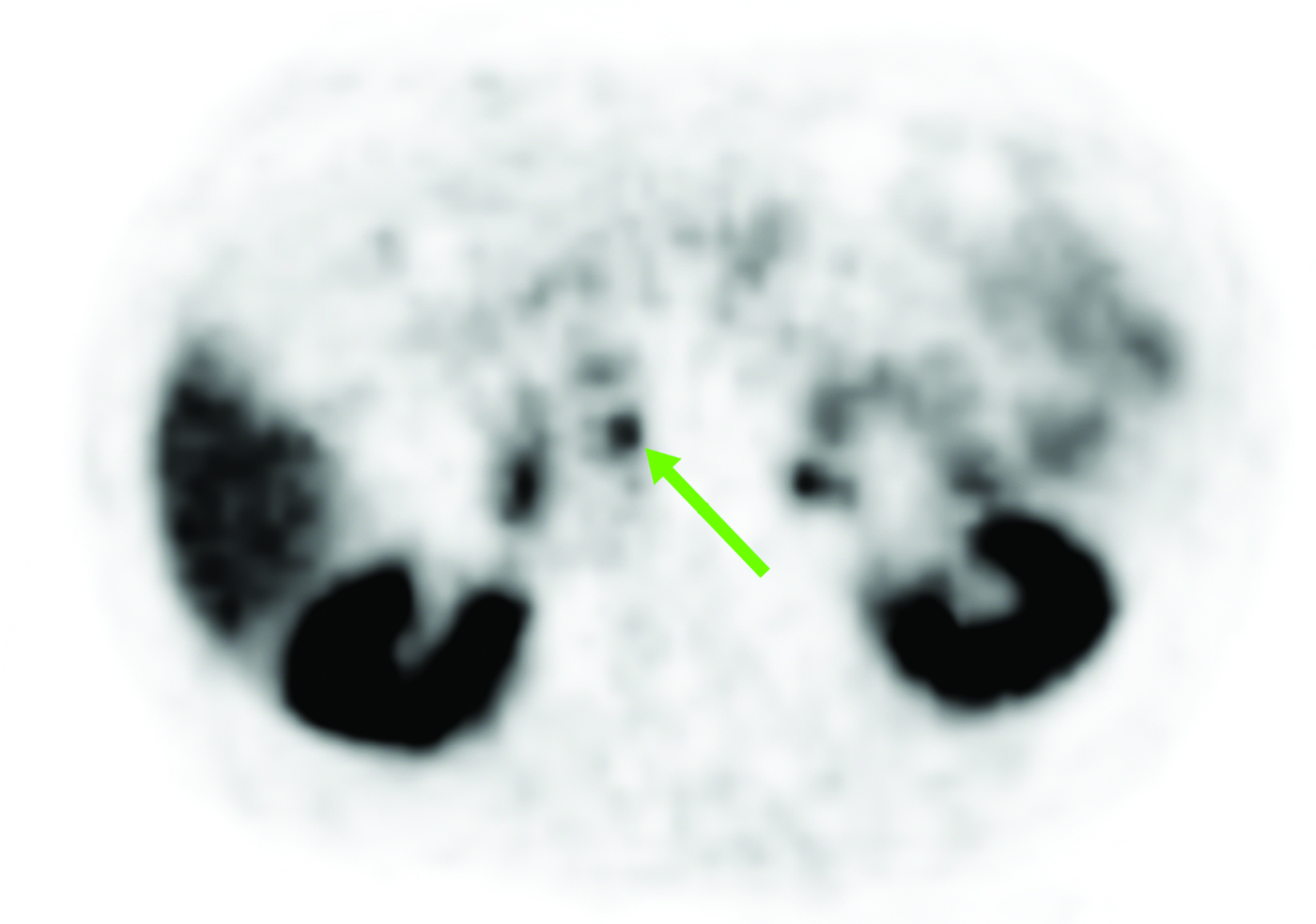
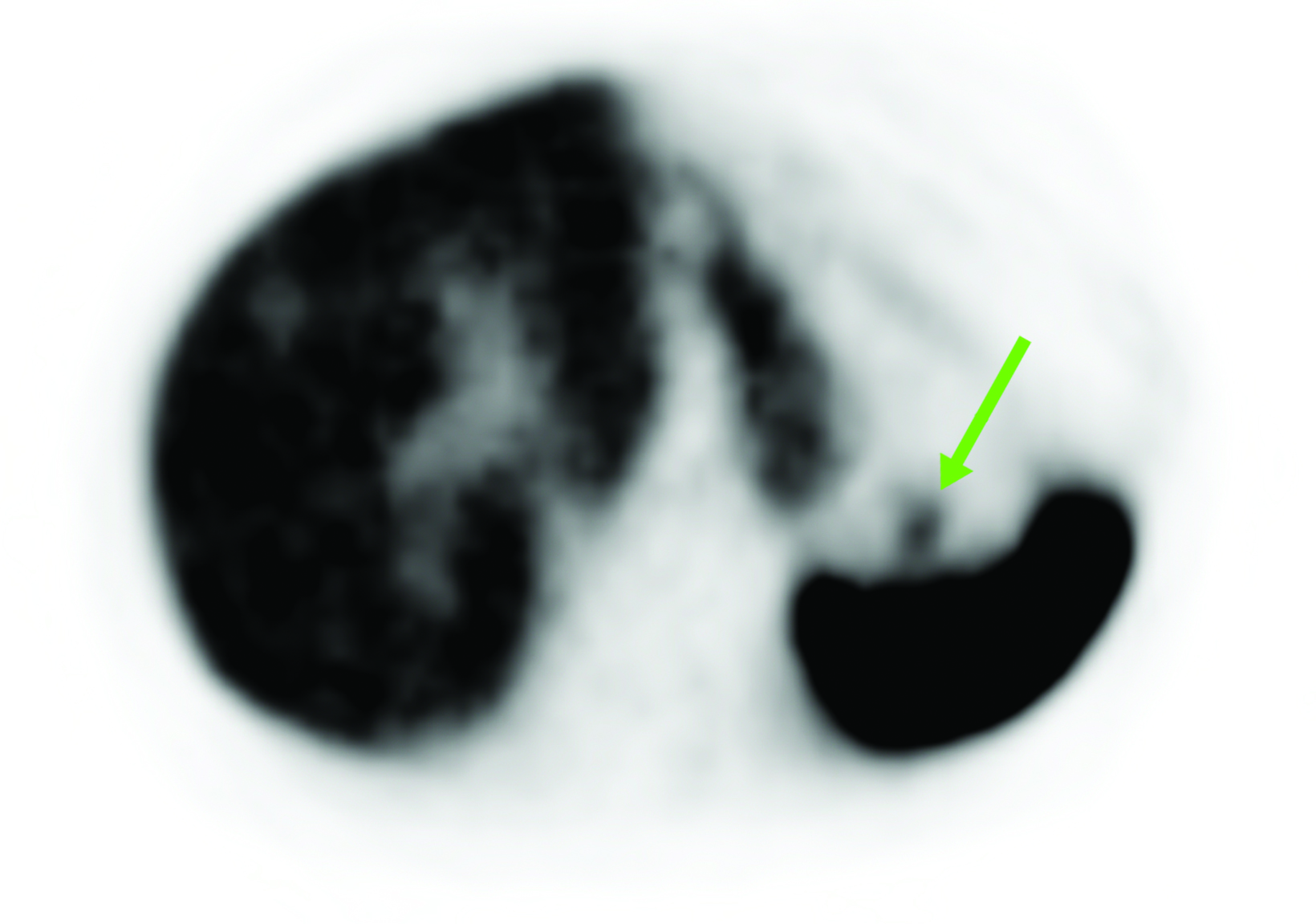
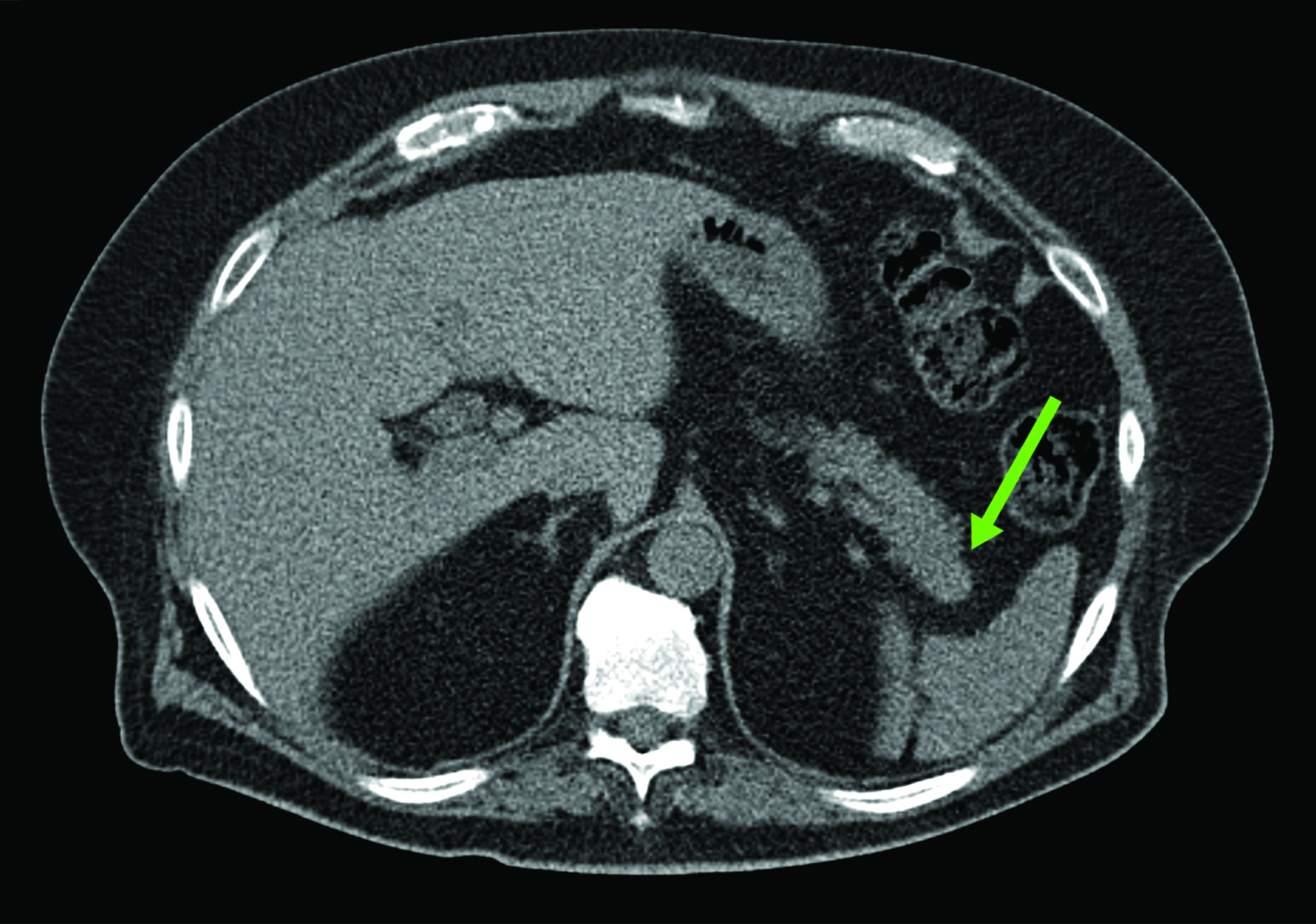
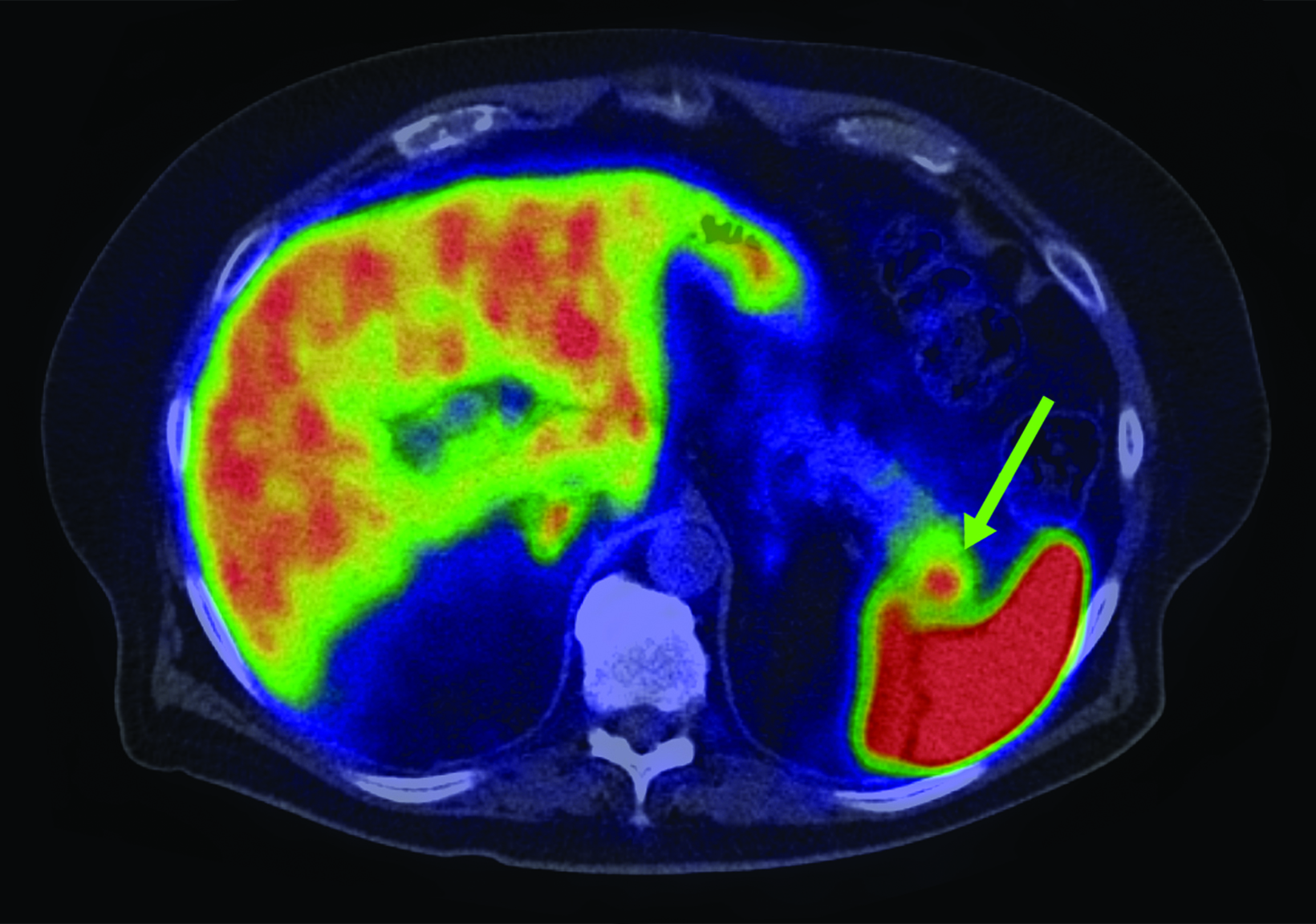
Although SSTR radiotracers are not known to localize to the pancreatic tail to the same degree as the uncinate process, variability in this region could similarly lead to diagnostic uncertainty. Focal islet cell hyperplasia has been reported as a possible mechanism causing increased SSTR expression in the pancreatic tail in patients with high physiologic activity (Figure 3).29 A study in 35 patients by Delbeke et al found that physiologic uptake within the pancreatic tail is typically equal or less than that of the liver.29 Therefore, quantification with SUVs, as well as correlation with CT or MRI, could play a role in discriminating normal and malignant activity in the pancreatic tail.
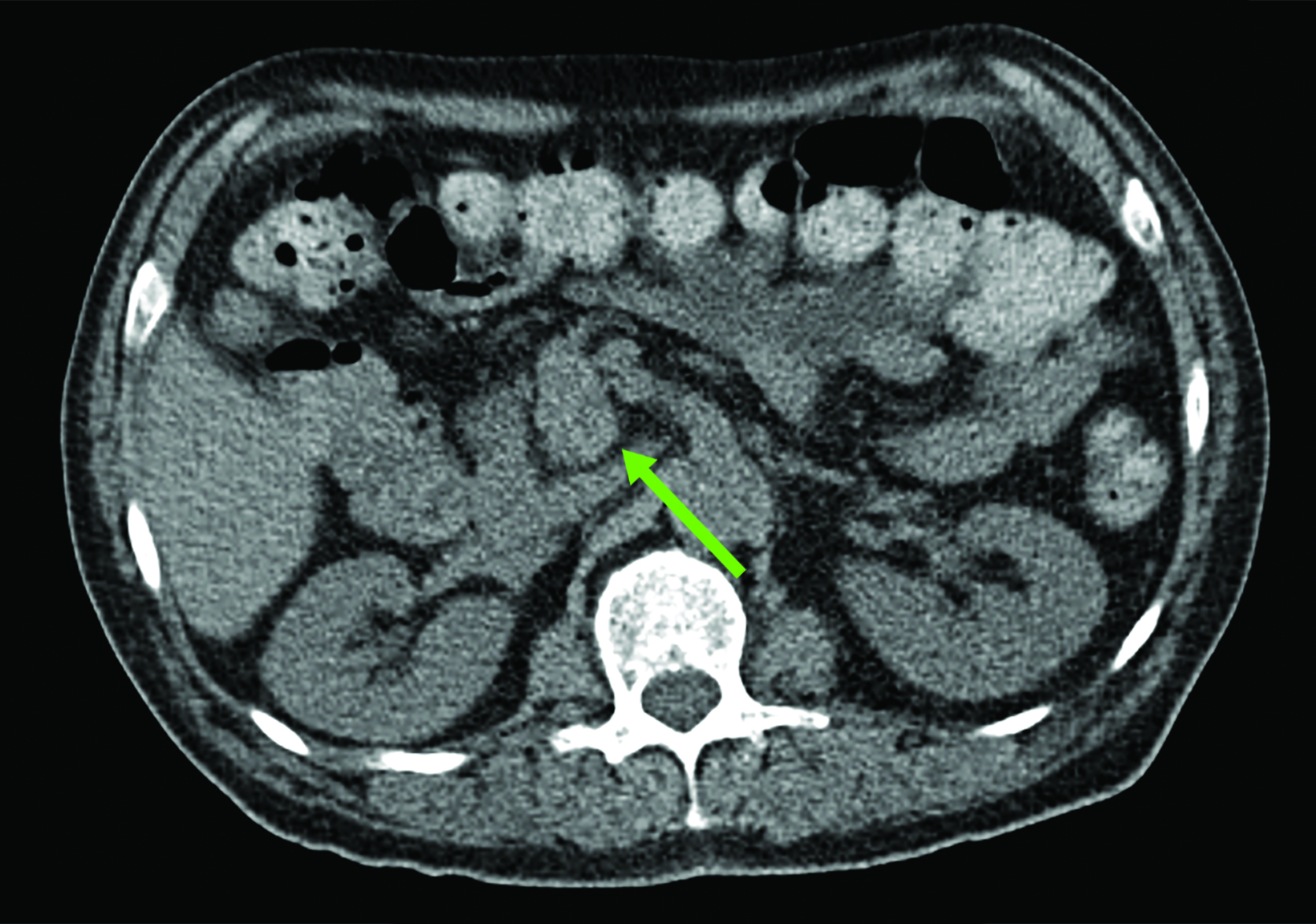
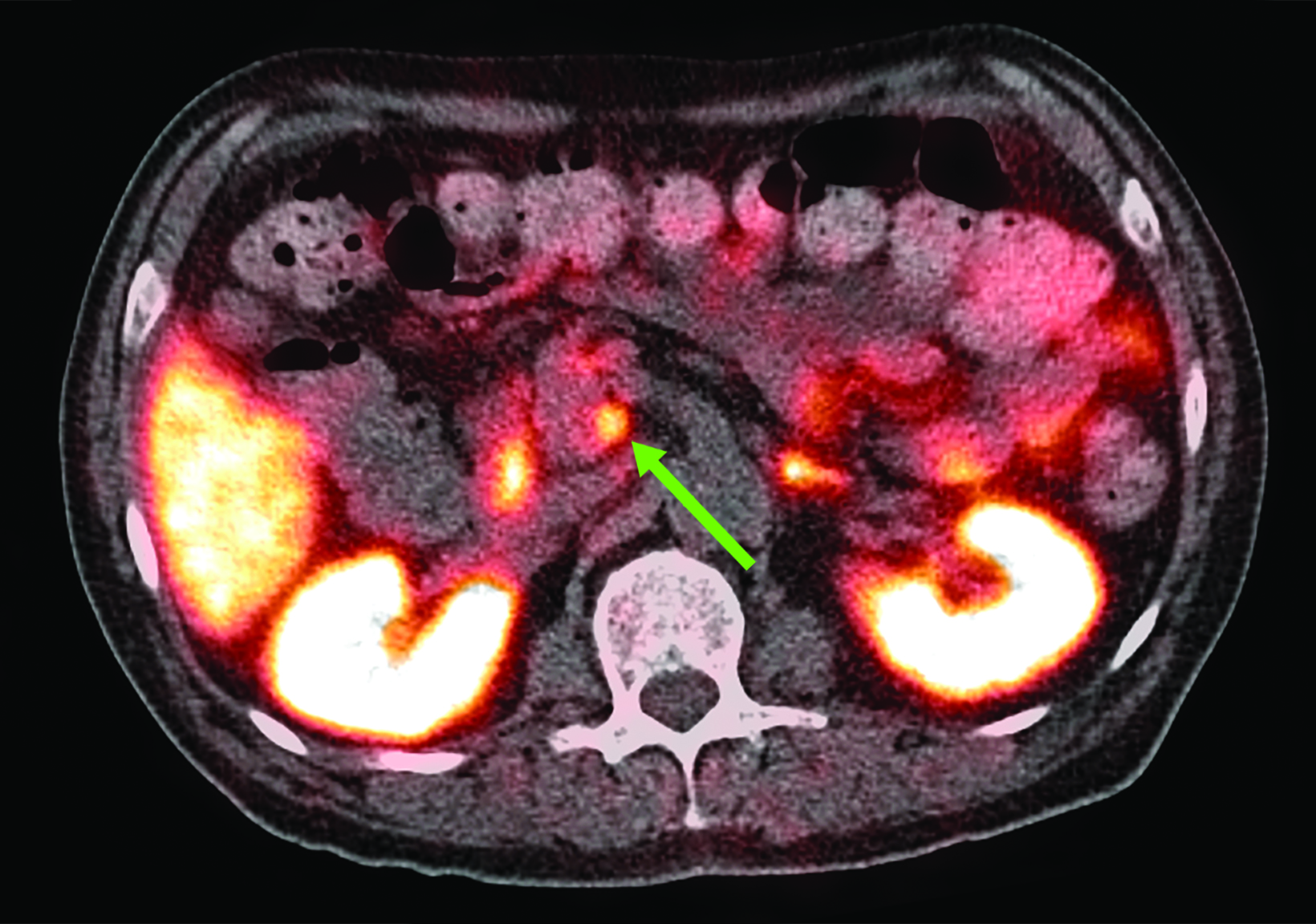
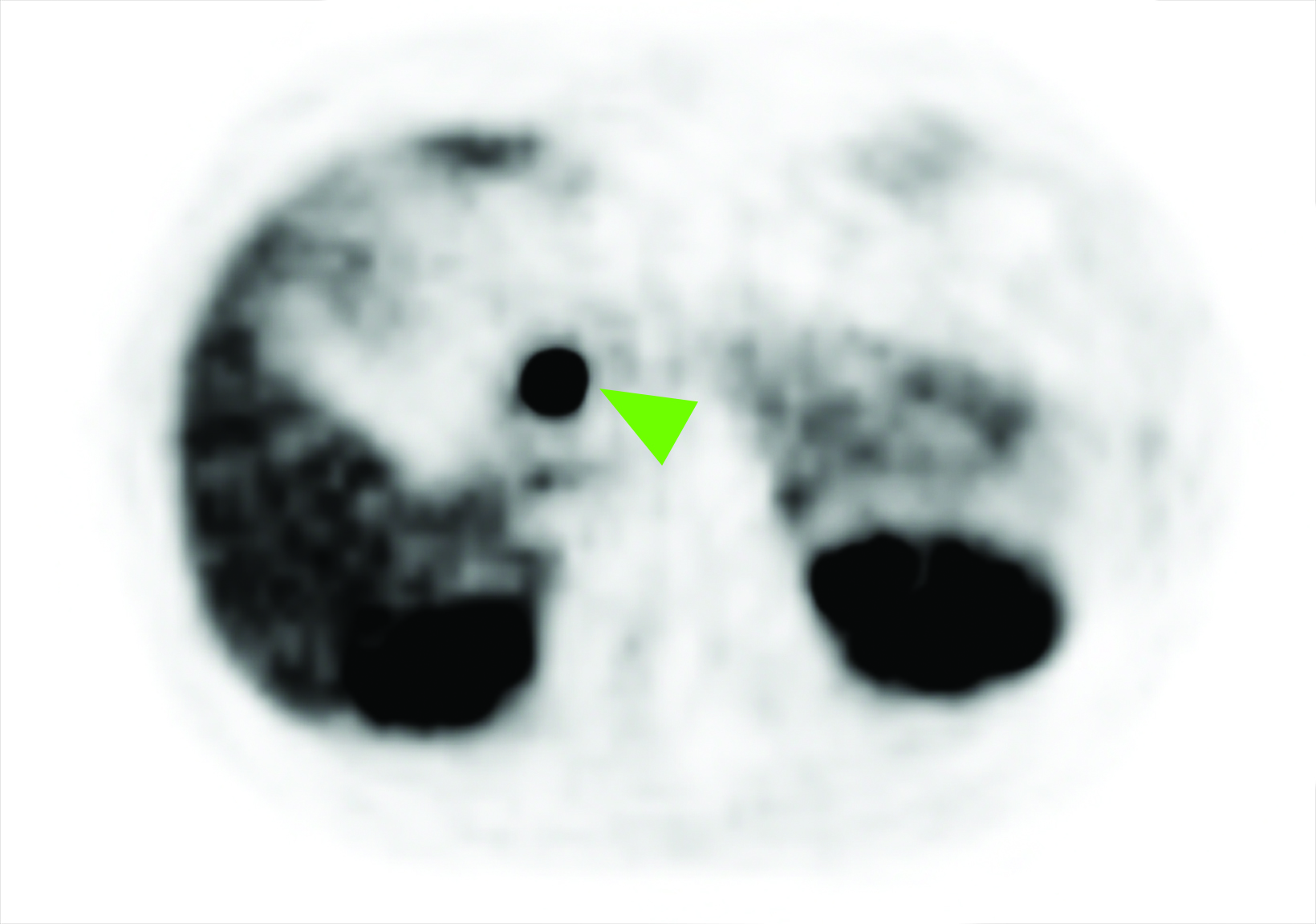
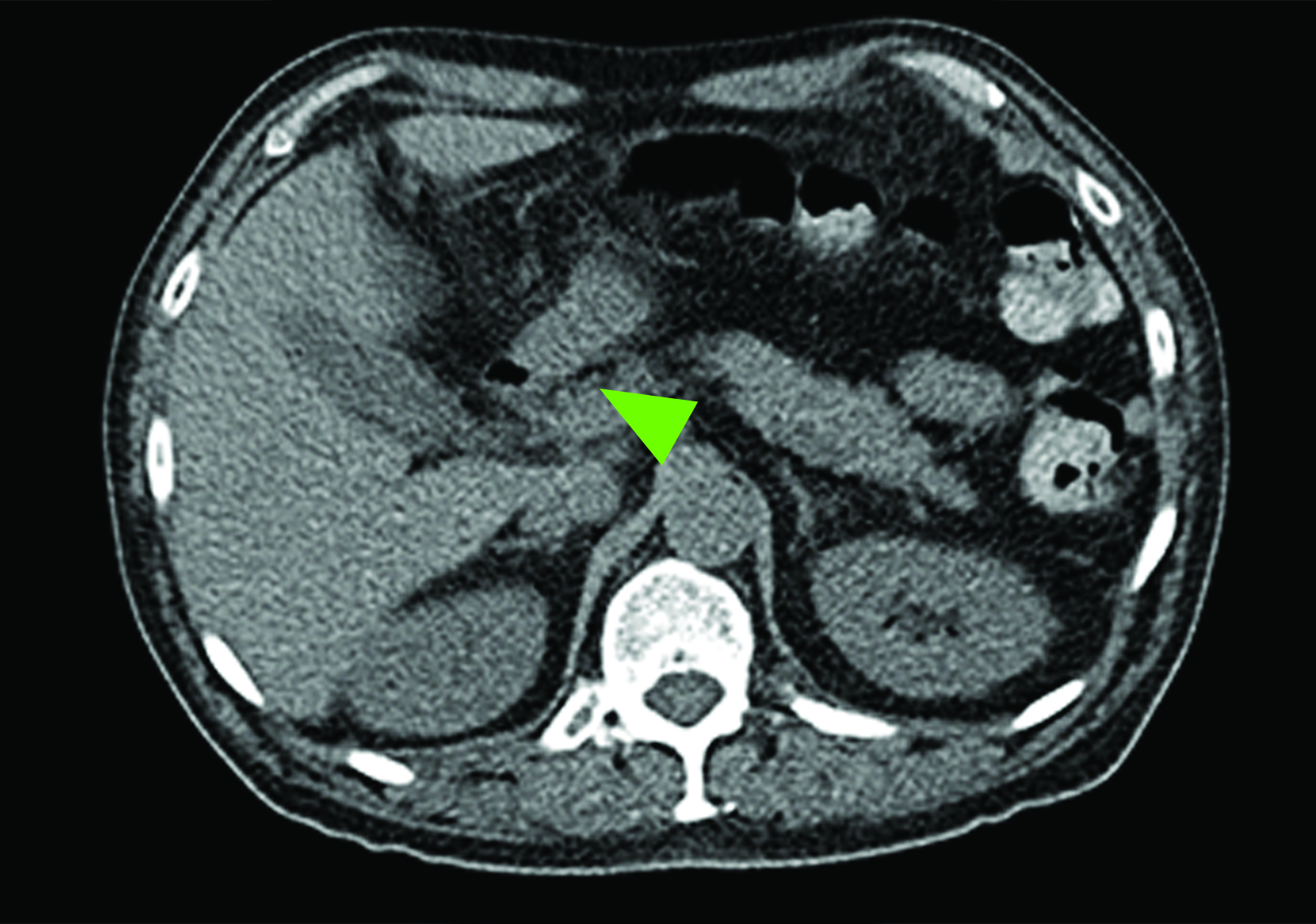
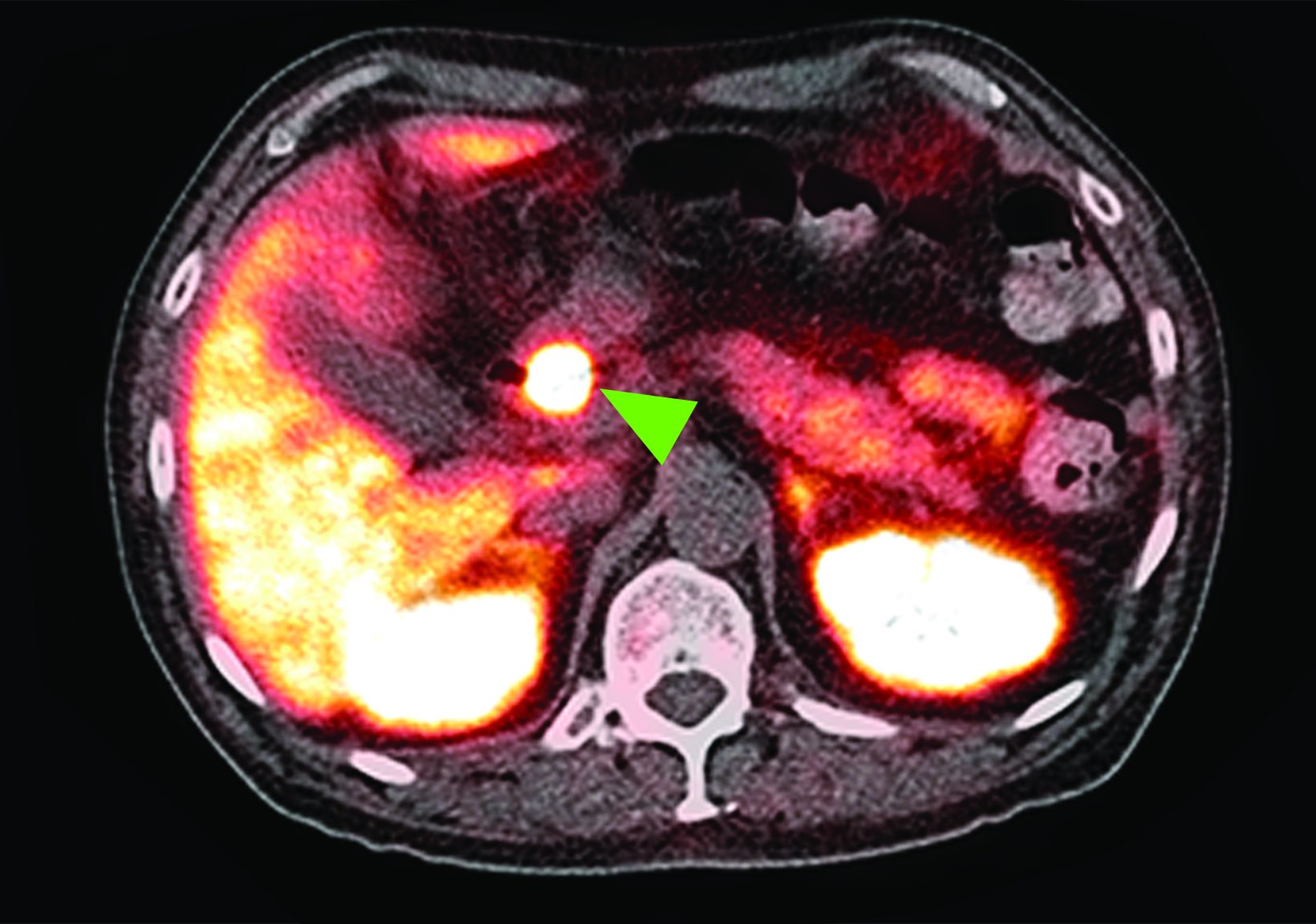
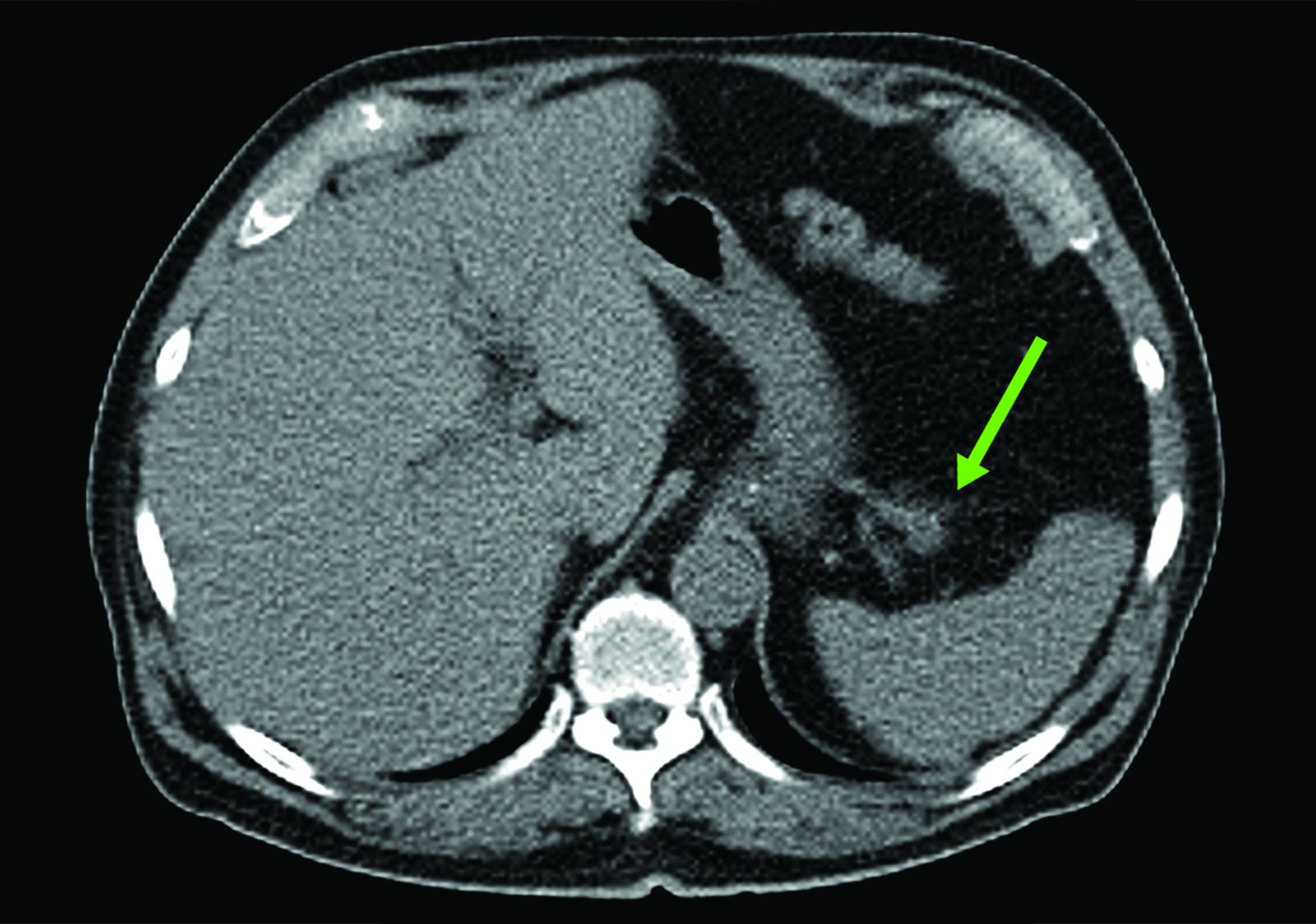
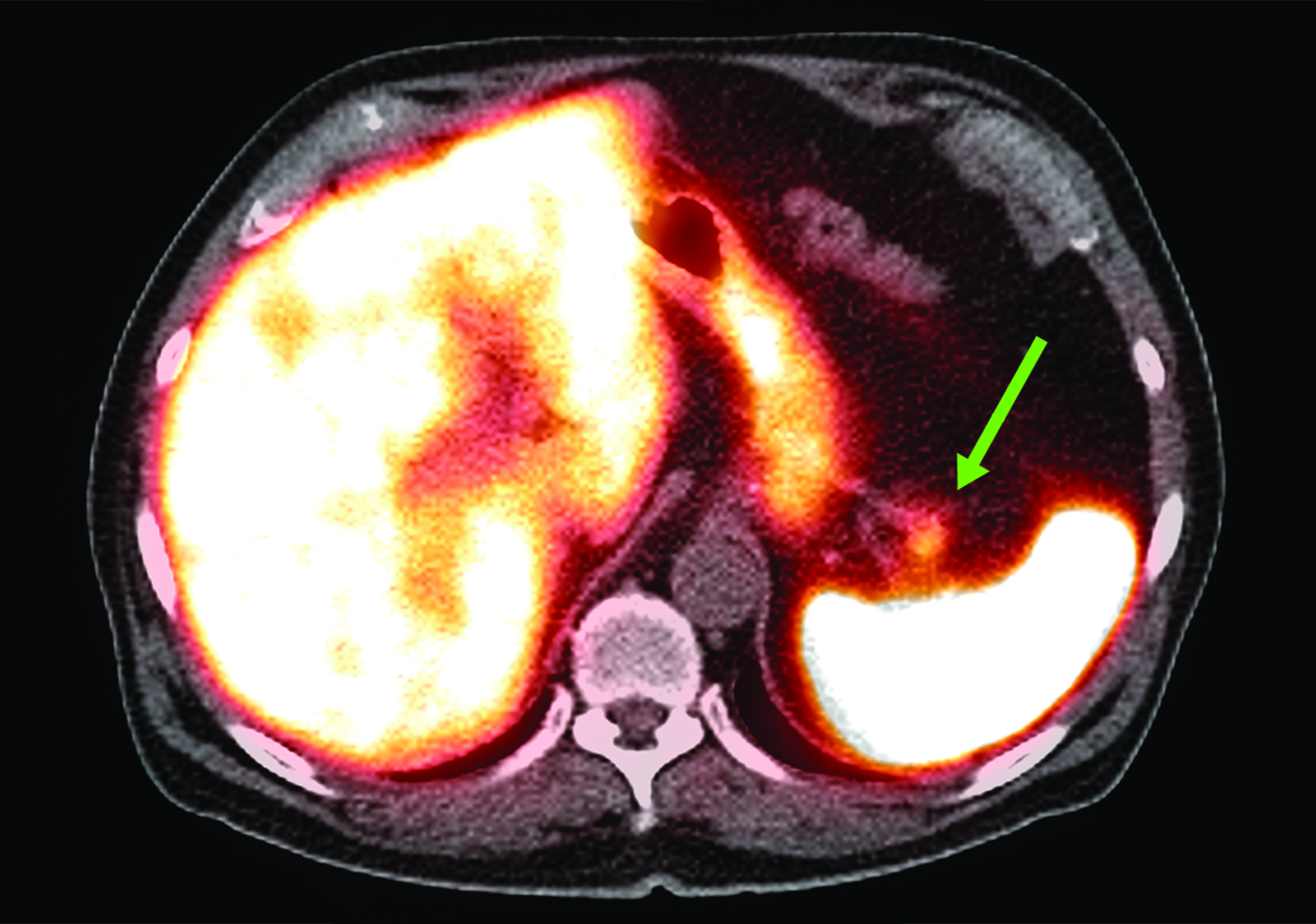
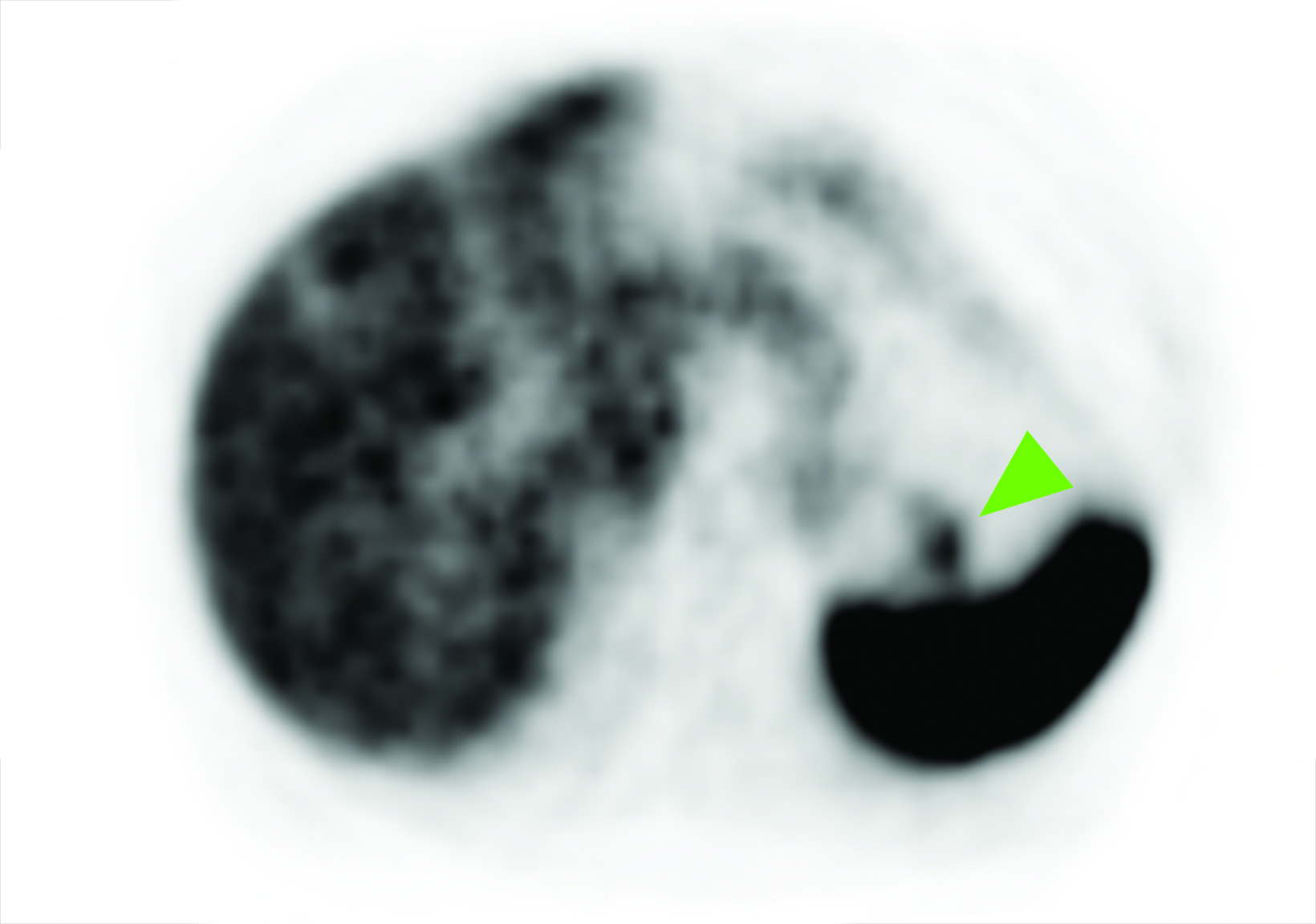
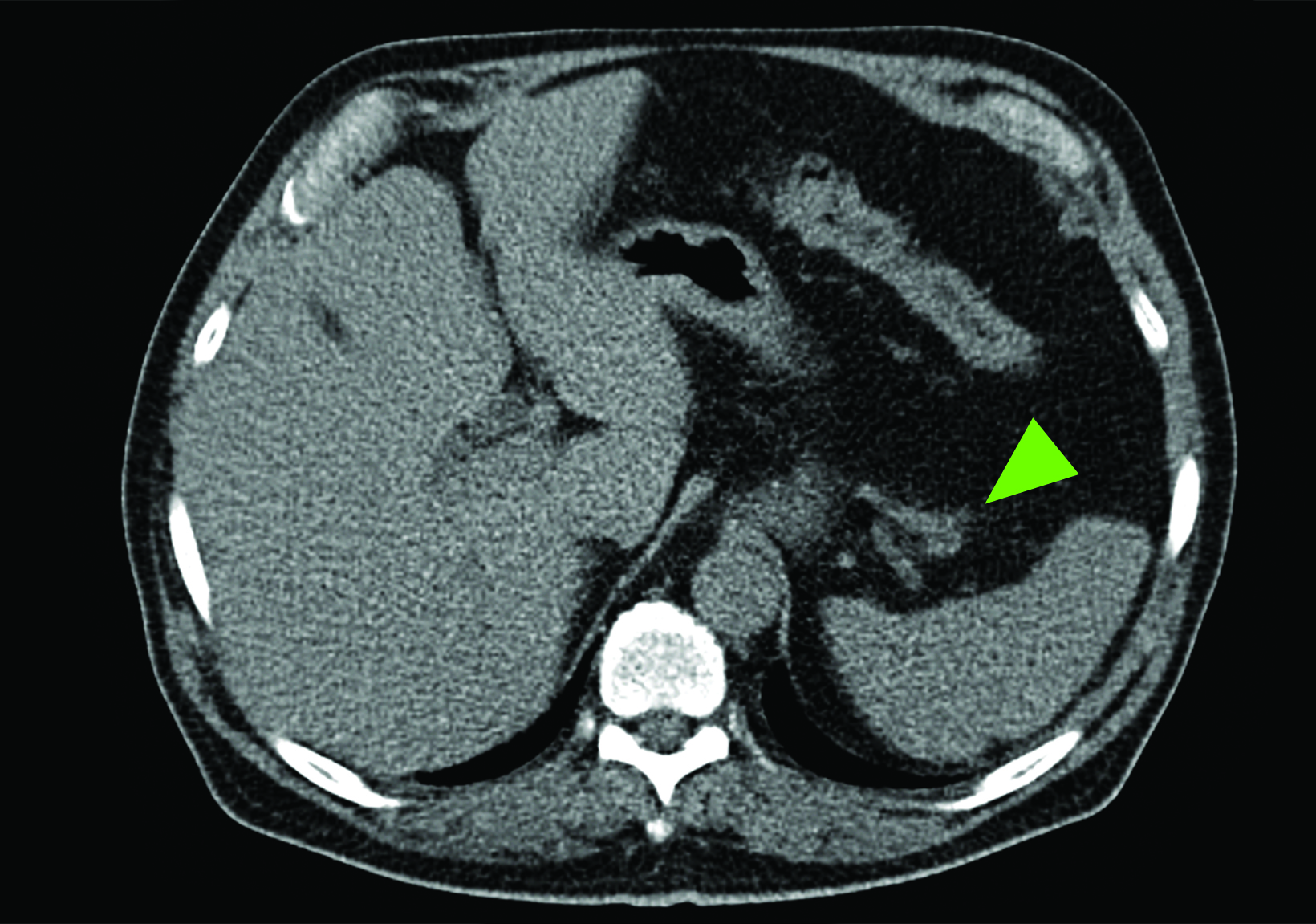
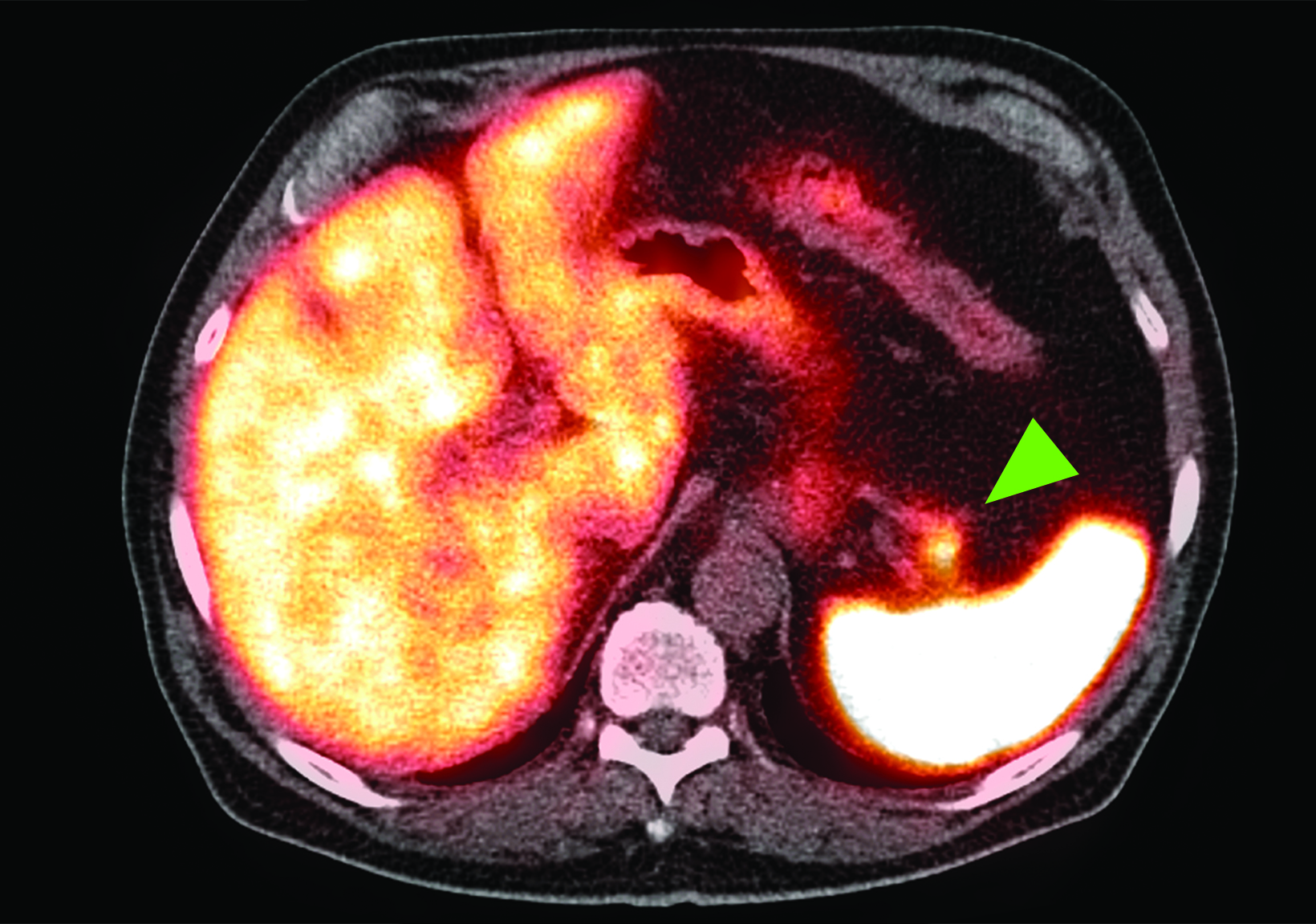
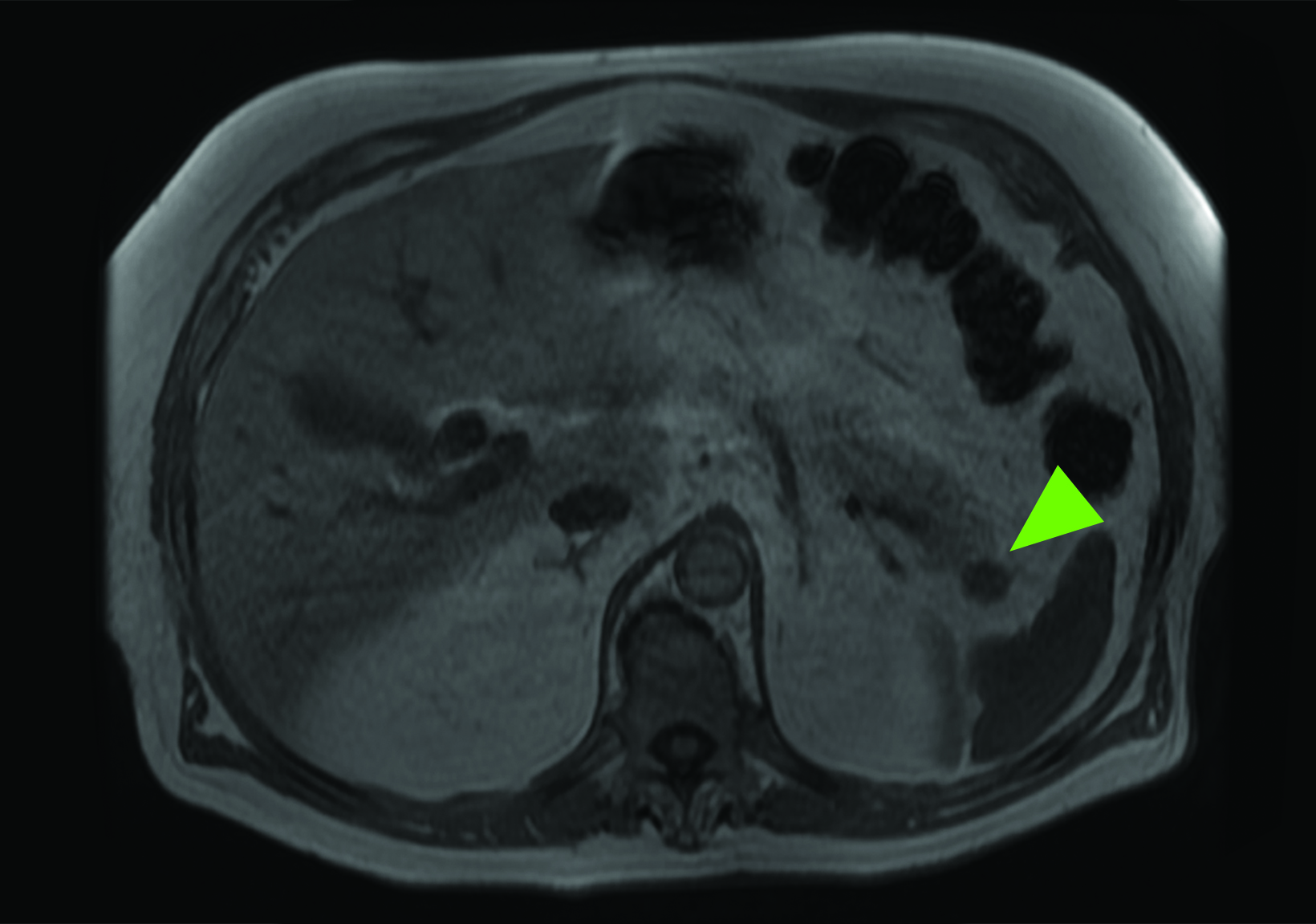
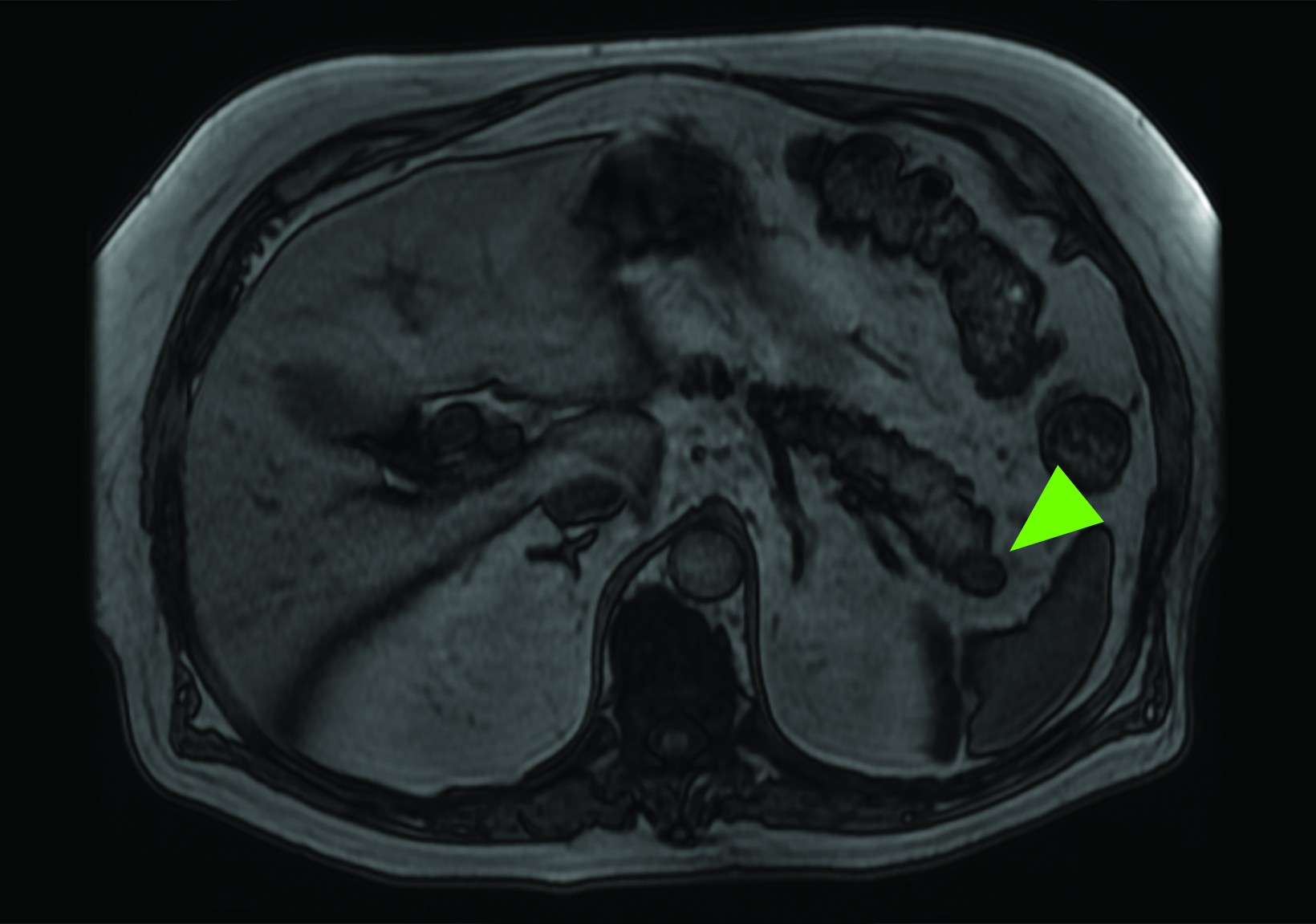
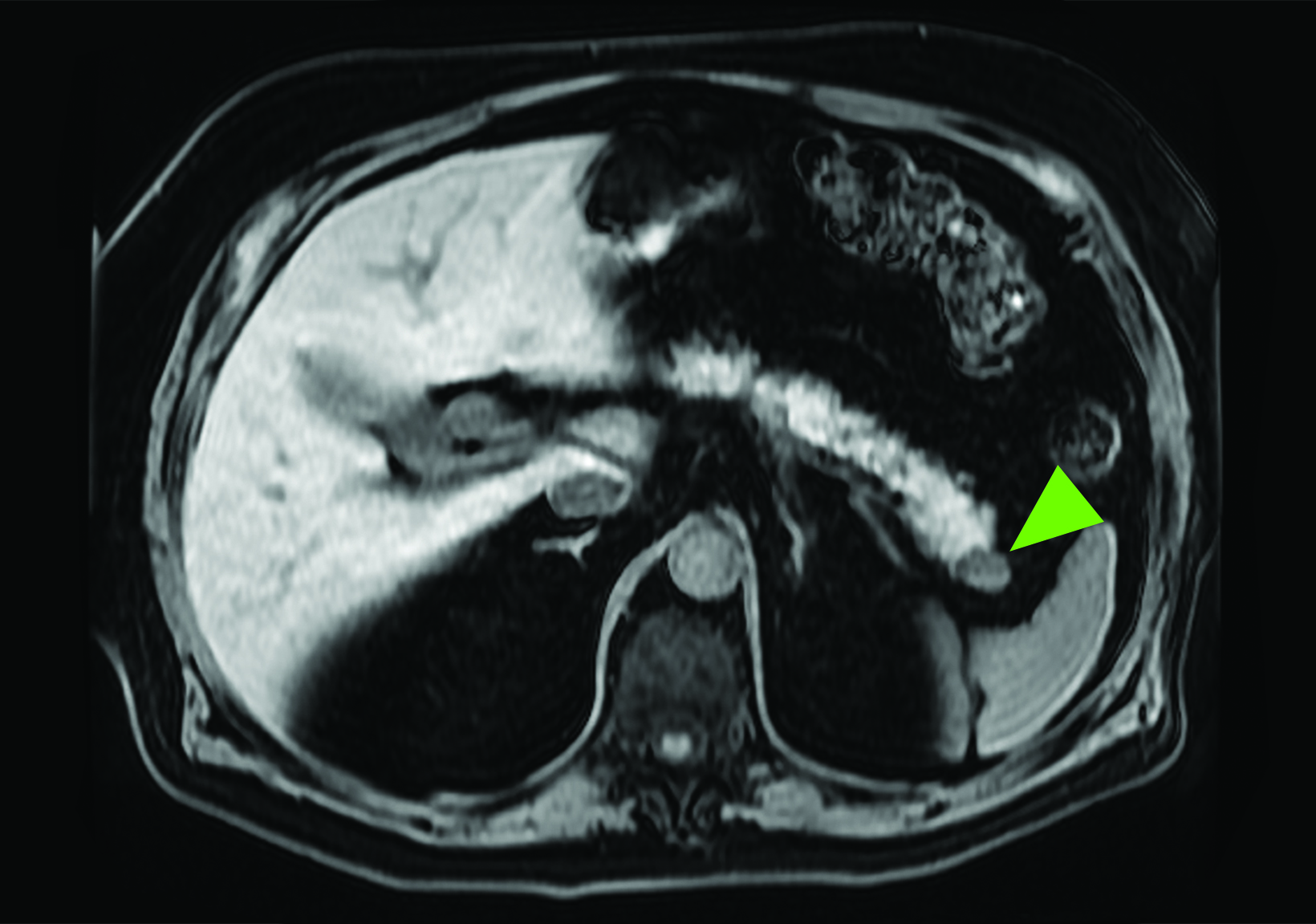
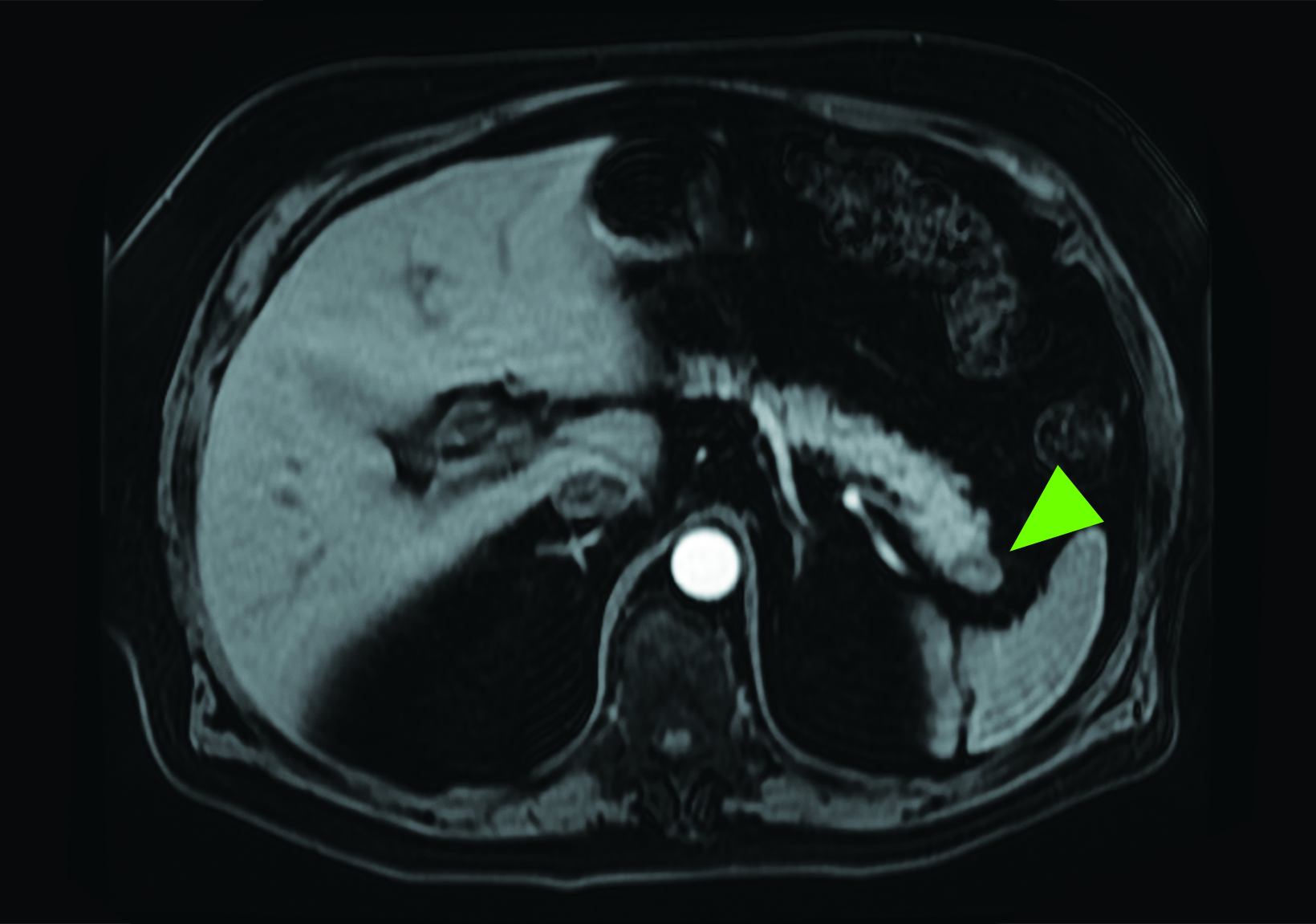
Related to the intense physiologic uptake demonstrated by the spleen, accessory spleen SSTR activity is similarly high, which can acquire an appearance suspicious for NET involvement, especially if intrapancreatic (Figure 4).30-32 Splenosis, often occurring after trauma or splenectomy, represents another atypical splenic tissue distribution that can resemble malignant activity.33 By assessing whether 99mTc-labeled sulfur colloid or heat-denatured red blood cell scintigraphy is able to confirm the presence of splenic tissue rather than tumor, misdiagnosis and subsequent high-risk intervention are potentially avoidable.34
Imaging of Inflammation
White blood cells such as macrophages are known to express SSTR2, resulting in low grade uptake associated with inflammation. As a result, a variety of inflammatory conditions imaged by SSTR PET/CT have been reported in the literature, including endometriosis, large vessel vasculitis, idiopathic pulmonary fibrosis, and pulmonary tuberculosis.35-39
SSTR PET has shown preliminary success in assessing systemic sarcoidosis. 68Ga-DOTATOC PET/CT was compared to 67Ga scintigraphy in a study of 20 sarcoidosis patients by Nobashi et al, who noted 68Ga-DOTATOC-positive lesions in 19 patients and 67Ga-positive lesions in 17 patients.40 These results not only indicate that SSTR PET is a feasible method of assessing sarcoidosis-related inflammation, but also that its performance may be similar or better than that of 67Ga, which is an established marker of infection and inflammation.
A study which corroborates this finding used 68Ga-DOTANOC PET/CT in 39 patients and observed a sensitivity of 93% and a specificity of 83% in the diagnosis of sarcoidosis, associated with decreased activity after treatment with symptomatic improvement.41 The described correlation between imaging and clinical improvement thus provides convergent validity for this approach.
A case report showing focal intracranial 68Ga-DOTATATE in the cavernous sinus of a symptomatic patient suggests a role for SSTR PET/CT in neurosarcoidosis, which was confirmed via biopsy.42 In addition to new potential clinical indications for imaging, these early observations also indicate that presence of sarcoidosis could complicate image interpretation in patients undergoing assessment for SSTR-positive neoplasm.
In light of the growing role of 18F-fluorodeoxyglucose (FDG) PET/CT in cardiac sarcoidosis, experiences using SSTR PET/CT for the same indication have started to appear.43-48 A 2016 study by Gormsen et al compared PET/CT with 18F-FDG and 68Ga-DOTANOC in a prospective analysis of 19 patients with suspected cardiac sarcoidosis.49 The authors found that while 18F-FDG findings were inconclusive in 11 patients, there were no inconclusive 68Ga-DOTANOC studies. The overall diagnostic accuracy of 18F-FDG for cardiac sarcoidosis was reported as 79%, compared to an overall accuracy of 100% for68Ga-DOTANOC in this small cohort.
A more recent study published in 2021 by Bravo et al showed that in 13 subjects with suspected cardiac sarcoidosis, all of whom had positive 18F-FDG findings, only 7 subjects showed definite or probable abnormal cardiac uptake of 68Ga-DOTATATE.50 However, there was 100% concordance between 18F-FDG and 68Ga-DOTATATE in positive thoracic nodal involvement, suggesting that the role of SSTR PET in cardiac sarcoidosis is less clear compared to the stronger evidence supporting its use in systemic manifestations.
Atherosclerotic disease has also been shown to correlate with SSTR tracer activity due to the presence of macrophages, potentially allowing for the early identification of vulnerable plaques in patients with risk factors.51,52 By focusing on symptomatic carotid artery plaques in 10 patients planning to undergo carotid endarterectomy, Pedersen et al found that uptake of 64Cu-DOTATATE was higher in symptomatic plaques compared to the contralateral side.53 After analyzing gene expression in the plaque specimens, the investigators found that tracer activity was correlated with the presence of alternatively activated macrophages.
The advantage of using 68Ga-DOTATATE rather than 18F-FDG for atherosclerosis imaging was illustrated in a study that showed feasible coronary artery disease assessment in all 42 patients assessed with 68Ga-DOTATATE, which was not possible with 18F-FDG in most cases due to high adjacent myocardial activity.54 SSTR PET has also shown decreased inflammation in atheromatous plaques in response to medical intervention. Specifically, 22 subjects with type 2 diabetes were imaged with 68Ga-DOTATATE PET/CT before and 3 months after initiating atorvastatin therapy, with a resulting 31% decrease in target-to-background ratio.55 Similarly, 64Cu-DOTATATE coronary uptake was found to decrease after 26 weeks of liraglutide therapy in 30 patients with type 2 diabetes.56 Therefore, SSTR PET could help guide clinical decision making by identifying severity of disease and assessing efficacy of treatment.
Discussion
SSTR PET is currently being performed clinically for the primary purposes of staging and following NETs. Although the uptake mechanism of SSTR tracers is more specific than that of 18F-FDG, many nontumor sources of uptake are still present. Familiarity with normal tissues which naturally express SSTR and common variations such as elevated uptake in the pancreatic uncinate process is necessary to avoid interpretive error. Normal splenic anatomy would be unlikely to confuse a reader, but the presence of an accessory spleen in an ambiguous or deceptive location could result in a diagnostic dilemma, warranting identification of splenic tissue with scintigraphic techniques or correlation with additional anatomic imaging.
Splenosis would be of particular concern in patients having undergone resection of NET, which is sometimes accompanied by splenectomy in cases with pancreatic involvement. Subtle morphological signs of splenosis such as the presence of smooth, round nodules combined with knowledge of the patient’s treatment course may alert the reader to the possibility of splenosis rather than more concerning pathology such as peritoneal metastasis, additional testing would often be necessary given the lack of specificity of SSTR PET in such cases.
Similar to physiologic phenomena, benign and inflammatory disorders can hinder accurate interpretation if alternative explanations are not considered. For example, a focal 68Ga-DOTATATE-avid lesion in the cavernous sinus may appear classic for meningioma, but in rare instances this may instead represent neurosarcoidosis. SSTR-positive mediastinal or hilar lymph nodes may represent either nodal metastases or reactive, infectious, or inflammatory lymph nodes depending on exposures and co-morbidities.
Although the distinctive vascular pattern and low-level uptake associated with atherosclerosis would be unlikely to pose a diagnostic challenge, the strong association between SSTR activity and presence of activated macrophages in atherosclerotic plaques suggests a possible role in directing therapy.
Conclusion
SSTR PET imaging has quickly revolutionized diagnosis, treatment, and surveillance of NETs and other SSTR-expressing tumors. A nuanced understanding of tracer behavior is necessary for precise image interpretation and optimal utilization.
References
- Brazeau P, Vale W, Burgus R, et al Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179(4068):77-79.
- Shamsi BH, Chatoo M, Xu XK, Xu X, Chen XQ. Versatile functions of somatostatin and somatostatin receptors in the gastrointestinal system. Front Endocrinol (Lausanne). 2021;12:652363.
- Ferone D, Pivonello R, Kwekkeboom DJ, et al Immunohistochemical localization and quantitative expression of somatostatin receptors in normal human spleen and thymus: Implications for the in vivo visualization during somatostatin receptor scintigraphy. J Endocrinol Invest. 2012;35(5):528-534.
- Boy C, Heusner TA, Poeppel TD, et al 68Ga-DOTATOC PET/CT and somatostatin receptor (sst1-sst5) expression in normal human tissue: correlation of sst2 mRNA and SUVmax. Eur J Nucl Med Mol Imaging. 2011;38(7):1224-1236.
- Taniyama Y, Suzuki T, Mikami Y, Moriya T, Satomi S, Sasano H. Systemic distribution of somatostatin receptor subtypes in human: an immunohistochemical study. Endocr J. 2005;52(5):605-611.
- Reubi JC, Schar JC, Waser B, et al Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraph- ic and radiotherapeutic use. Eur J Nucl Med. 2000;27(3):273-282.
- Krenning EP, Bakker WH, Kooij PP, et al Somatostatin receptor scintigraphy with indium-111-DTPA-D-Phe-1-octreotide in man: metabolism, dosimetry and comparison with iodine-123-Tyr-3-octreotide. J Nucl Med. 1992;33(5):652-658.
- Hofman MS, Lau WF, Hicks RJ. Somatostatin receptor imaging with 68Ga DOTATATE PET/CT: clinical utility, normal patterns, pearls, and pitfalls in interpre- tation. Radiographics. 2015;35(2):500-516.
- Cascini GL, Cuccurullo V, Tamburrini O, Rotondo A, Mansi L. Peptide imaging with somatostatin analogues: more than cancer probes. Curr Radiopharm. 2013;6(1):36-40.
- Strosberg J, El-Haddad G, Wolin E, et al Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N Engl J Med. 2017;376(2):125-135.
- Reubi JC, Waser B, Horisberger U, et al. In vitro autoradiographic and in vivo scintigraphic localization of somatostatin receptors in human lymphatic tis- sue. Blood. 1993;82(7):2143-2151.
- hastry M, Kayani I, Wild D, et al Distribution pattern of 68Ga-DOTATATE in disease-free patients. Nucl Med Commun. 2010;31(12):1025-1032.
- Sandstrom M, Velikyan I, Garske-Roman U, et al Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54(10):1755-1759.
- Wild D, Bomanji JB, Benkert P, et al Comparison of 68Ga-DOTANOC and 68Ga-DOTATATE PET/CT within patients with gastroenteropancreatic neuroendo- crine tumors. J Nucl Med. 2013;54(3):364-372.
- Conti M, Eriksson L. Physics of pure and non-pure positron emitters for PET: a review and a discussion. EJNMMI Phys. 2016;3(1):8.
- Johnbeck CB, Knigge U, Loft A, et al. Head-to-head comparison of (64)Cu-DOTATATE and (68)Ga-DOTATOC PET/CT: a prospective study of 59 patients with neuroendocrine tumors. J Nucl Med. 2017;58(3):451-457.
- Tabacchi E, Fortunati E, Argalia G, et al. [68Ga]Ga-DOTANOC Uptake at pancreatic head/uncinate process: is it a persistent diagnostic pitfall over time? Cancers (Basel). 2022;14(14).
- Mapelli P, Tam HH, Sharma R, Aboagye EO, Al-Nahhas A. Frequency and significance of physiological versus pathological uptake of 68Ga-DOTATATE in the pancreas: validation with morphological imaging. Nucl Med Commun. 2014;35(6):613-619.
- Lakhotia R, Jhawar S, Malayeri AA, Millo C, Del Rivero J, Ahlman MA. Incidental 68Ga-DOTATATE uptake in the pancreatic head: A case report and a unique opportunity to improve clinical care. Medicine (Baltimore). 2020;99(22):e20197.
- Brabander T, Teunissen J, Kwekkeboom D. Physiological uptake in the pancreatic head on somatostatin receptor scintigraphy using [111In-DTPA]octreotide: incidence and mechanism. Clin Nucl Med. 2017;42(1):15-19.
- Ait Boudaoud A, Verges B, Petit JM, Tatulashvili S, Cochet A, Humbert O. Uptake in the pancreatic uncinate process on the 111In-octreotide scintigraphy: How to distinguish physiological from pathological uptake? Nucl Med Commun. 2017;38(9):737-743.
- Krausz Y, Rubinstein R, Appelbaum L, et al. Ga-68 DOTA-NOC uptake in the pancreas: pathological and physiological patterns. Clin Nucl Med. 2012;37(1):57-62.
- Jacobsson H, Larsson P, Jonsson C, Jussing E, Gryback P. Normal uptake of 68Ga-DOTA-TOC by the pancreas uncinate process mimicking malignancy at somatostatin receptor PET. Clin Nucl Med. 2012;37(4):362-365.
- Kunikowska J, Krolicki L, Pawlak D, Zerizer I, Mikolajczak R. Semiquantitative analysis and characterization of physiological biodistribution of (68)Ga- DOTA-TATE PET/CT. Clin Nucl Med. 2012;37(11):1052-1057.
- Castellucci P, Pou Ucha J, Fuccio C, et al Incidence of increased 68Ga-DOTANOC uptake in the pancreatic head in a large series of extrapancreatic NET patients studied with sequential PET/CT. J Nucl Med. 2011;52(6):886-890.
- Al-Ibraheem A, Bundschuh RA, Notni J, et al. Focal uptake of 68Ga-DOTATOC in the pancreas: pathological or physiological correlate in patients with neuro-endocrine tumours? Eur J Nucl Med Mol Imaging. 2011;38(11):2005-2013.
- >Wang X, Zielinski MC, Misawa R, et al Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One. 2013;8(1):e55501.
- Kroiss A, Putzer D, Decristoforo C, et al. 68Ga-DOTA-TOC uptake in neuroendocrine tumour and healthy tissue: differentiation of physiological uptake and pathological processes in PET/CT. Eur J Nucl Med Mol Imaging. 2013;40(4):514-523.
- >Delbeke D, Newman G, Deppen S, et al. 68Ga-DOTATATE: Significance of uptake in the tail of the pancreas in patients without lesions. Clin Nucl Med. 2019;44(11):851-854.
- Pang Y, Meng T, Shang Q, Chen Z, Chen H. 68Ga-Exendin-4 PET/CT differentiates insulinoma from accessory spleen in a patient presenting indeterminate MRI and 68Ga-DOTATATE PET/CT findings. Clin Nucl Med. 2022;47(3):265-267.
- >Lancellotti F, Sacco L, Cerasari S, et al Intrapancreatic accessory spleen false positive to 68Ga-Dotatoc: case report and literature review. World J Surg Oncol. 2019;17(1):117.
- >Bhure U, Metzger J, Keller FA, et al Intrapancreatic Accessory Spleen Mimicking Neuroendocrine Tumor on 68Ga-DOTATATE PET/CT. Clin Nucl Med. 2015;40(9):744-745.
- >Sachawars E, Lin M, Sidhom GE. Jumping the gun: traumatic splenosis mimicking 68-gallium-dotatate avid neuroendocrine tumour. ANZ J Surg. 2020;90(12):E217-E218.
- Gezer E, Cetinarslan B, Karakaya D, et al. Differentiation of insulinoma from accessory spleen by (99m)Tc-labelled heat-denaturated red blood cell scintigraphy: case report. BMC Endocr Disord. 2021;21(1):6.
- >Fastrez M, Artigas C, Sirtaine N, et al. Value of the (68)Ga-DOTATATE PET-CT in the diagnosis of endometriosis. A pilot study. Eur J Obstet Gynecol Reprod Biol. 2017;212:69-74.
- Corovic A, Wall C, Nus M, et al. Somatostatin receptor PET/MR imaging of inflammation in patients with large vessel vasculitis and atherosclerosis. J Am Coll Cardiol. 2023;81(4):336-354.
- Tarkin JM, Wall C, Gopalan D, et al. Novel approach to imaging active Takayasu arteritis using somatostatin receptor positron emission tomography/magnetic resonance imaging. Circ Cardiovasc Imaging. 2020;13(6):e010389.
- Ambrosini V, Zompatori M, De Luca F, et al. 68Ga-DOTANOC PET/CT allows somatostatin receptor imaging in idiopathic pulmonary fibrosis: preliminary results. J Nucl Med. 2010;51(12):1950-1955.
- Naftalin CM, Leek F, Hallinan J, et al. Comparison of 68Ga-DOTANOC with 18F-FDG using PET/MRI imaging in patients with pulmonary tuberculosis. Sci Rep. 2020;10(1):14236.
- >Nobashi T, Nakamoto Y, Kubo T, et al. The utility of PET/CT with (68)Ga-DOTATOC in sarcoidosis: comparison with (67)Ga-scintigraphy. Ann Nucl Med. 2016;30(8):544-552.
- Sharma S, Singh AD, Sharma SK, Tripathi M, Das CJ, Kumar R. Gallium-68 DOTA-NOC PET/CT as an alternate predictor of disease activity in sarcoidosis. Nucl Med Commun. 2018;39(8):768-778.
- >Unterrainer M, Ruf V, Ilhan H, et al. Teaching neuroImages: advanced imaging of neurosarcoidosis with (68)Ga-DOTATATE PET/CT. Neurology. 2019;92(21):e2512-e2513.
- Vachatimanont S, Kunawudhi A, Promteangtrong C, Chotipanich C. Benefits of [(68)Ga]-DOTATATE PET-CT comparable to [(18)F]-FDG in patient with sus- pected cardiac sarcoidosis. J Nucl Cardiol. 2022;29(1):381-383.
- Imperiale A, Poindron V, Martinez M, Ohlmann P, Schindler TH, El Ghannudi S. 68Ga-DOTATOC PET for treatment efficacy evaluation of cardiac sarcoidosis. Clin Nucl Med. 2020;45(9):e416-e418.
- Pizarro C, Kluenker F, Dabir D, et al. Cardiovascular magnetic resonance imaging and clinical performance of somatostatin receptor positron emission tomography in cardiac sarcoidosis. ESC Heart Fail. 2018;5(2):249-261.
- >Passah A, Kaushik P, Patel C, Parakh N. Gallium-68 DOTANOC scan in a patient with suspected cardiac sarcoidosis. J Nucl Cardiol. 2018;25(6):2177-2178.
- Lapa C, Reiter T, Kircher M, et al. Somatostatin receptor based PET/CT in patients with the suspicion of cardiac sarcoidosis: an initial comparison to cardiac MRI. Oncotarget. 2016;7(47):77807-77814.
- Reiter T, Werner RA, Bauer WR, Lapa C. Detection of cardiac sarcoidosis by macrophage-directed somatostatin receptor 2-based positron emission tomography/computed tomography. Eur Heart J. 2015;36(35):2404.
- Gormsen LC, Haraldsen A, Kramer S, Dias AH, Kim WY, Borghammer P. A dual tracer (68)Ga-DOTANOC PET/CT and (18)F-FDG PET/CT pilot study for detection of cardiac sarcoidosis. EJNMMI Res. 2016;6(1):52.
- Bravo PE, Bajaj N, Padera RF, et al Feasibility of somatostatin receptor-targeted imaging for detection of myocardial inflammation: A pilot study. J Nucl Cardiol. 2021;28(3):1089-1099.
- Jensen JK, Madsen JS, Jensen MEK, Kjaer A, Ripa RS. [(64)Cu]Cu-DOTATATE PET metrics in the investigation of atherosclerotic inflammation in humans. J Nucl Cardiol. 2023;30(3):986-1000.
- >Lee R, Kim J, Paeng JC, et al. Measurement of (68)Ga-DOTATOC uptake in the thoracic aorta and its correlation with cardiovascular risk. Nucl Med Mol Imaging. 2018;52(4):279-286.
- Pedersen SF, Sandholt BV, Keller SH, et al. 64Cu-DOTATATE PET/MRI for detection of activated macrophages in carotid atherosclerotic plaques: studies in patients undergoing endarterectomy. Arterioscler Thromb Vasc Biol. 2015;35(7):1696-1703.
- Tarkin JM, Joshi FR, Evans NR, et al. Detection of atherosclerotic inflammation by (68)Ga-DOTATATE PET compared to [(18)F]FDG PET imaging. J Am Coll Cardiol. 2017;69(14):1774-1791.
- Oostveen RF, Kaiser Y, Stahle MR, et al. Atorvastatin lowers (68)Ga-DOTATATE uptake in coronary arteries, bone marrow and spleen in individuals with type 2 diabetes. Diabetologia. 2023.
- Jensen JK, Zobel EH, von Scholten BJ, et al. Effect of 26 weeks of liraglutide treatment on coronary artery inflammation in type 2 diabetes quantified by [(64) Cu]Cu-DOTATATE PET/CT: results from the LIRAFLAME trial. Front Endocrinol (Lausanne). 2021;12:790405.
Citation
WY R, JS K,. Somatostatin Receptor PET Imaging of Physiologic and Benign Processes: Implications for Image Interpretation, Avoiding Pitfalls, and Clinical Applications. Supplement to Applied Radiology. 2024;(1):36-43.
January 25, 2024