Schistosoma Bladder Infection
Images
Case Summary
A child was referred to the nephrology clinic with several years’ history of microscopic hematuria and a single episode of sterile pyuria. The patient reported no other associated symptoms and appeared well overall. Of note, the patient had visited a country in Africa, where they played and bathed in rivers. Their cousins also developed microscopic hematuria, which resolved after being administered an unknown medication.
Urinalysis was remarkable for proteinuria and significant blood but negative for leukocyte esterase and nitrites. Although the white blood cell count was normal, eosinophils were elevated at 23.7%. There were no ova or parasites in the stool. Serology was positive for Schistosoma IgG.
Imaging Findings
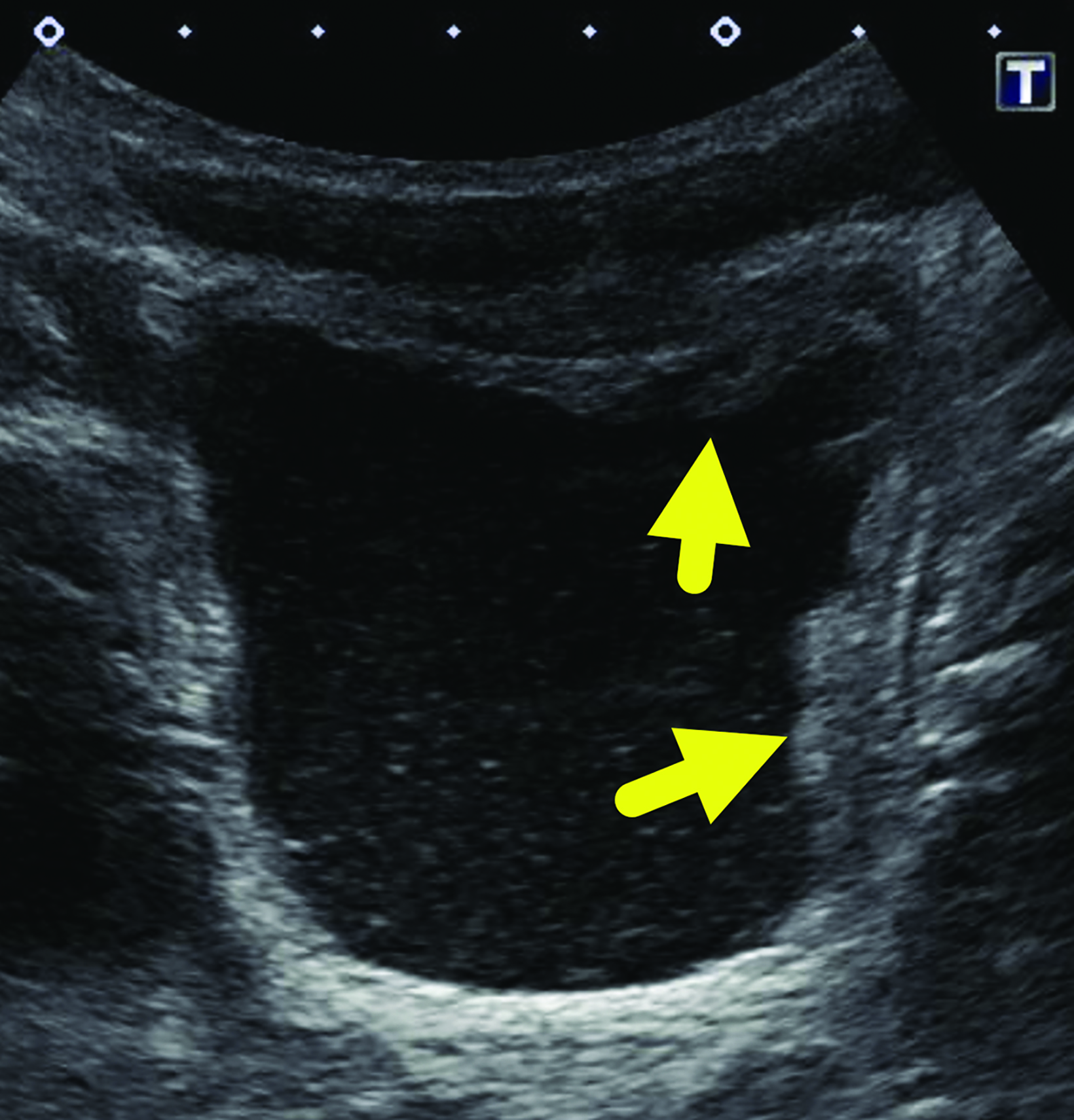
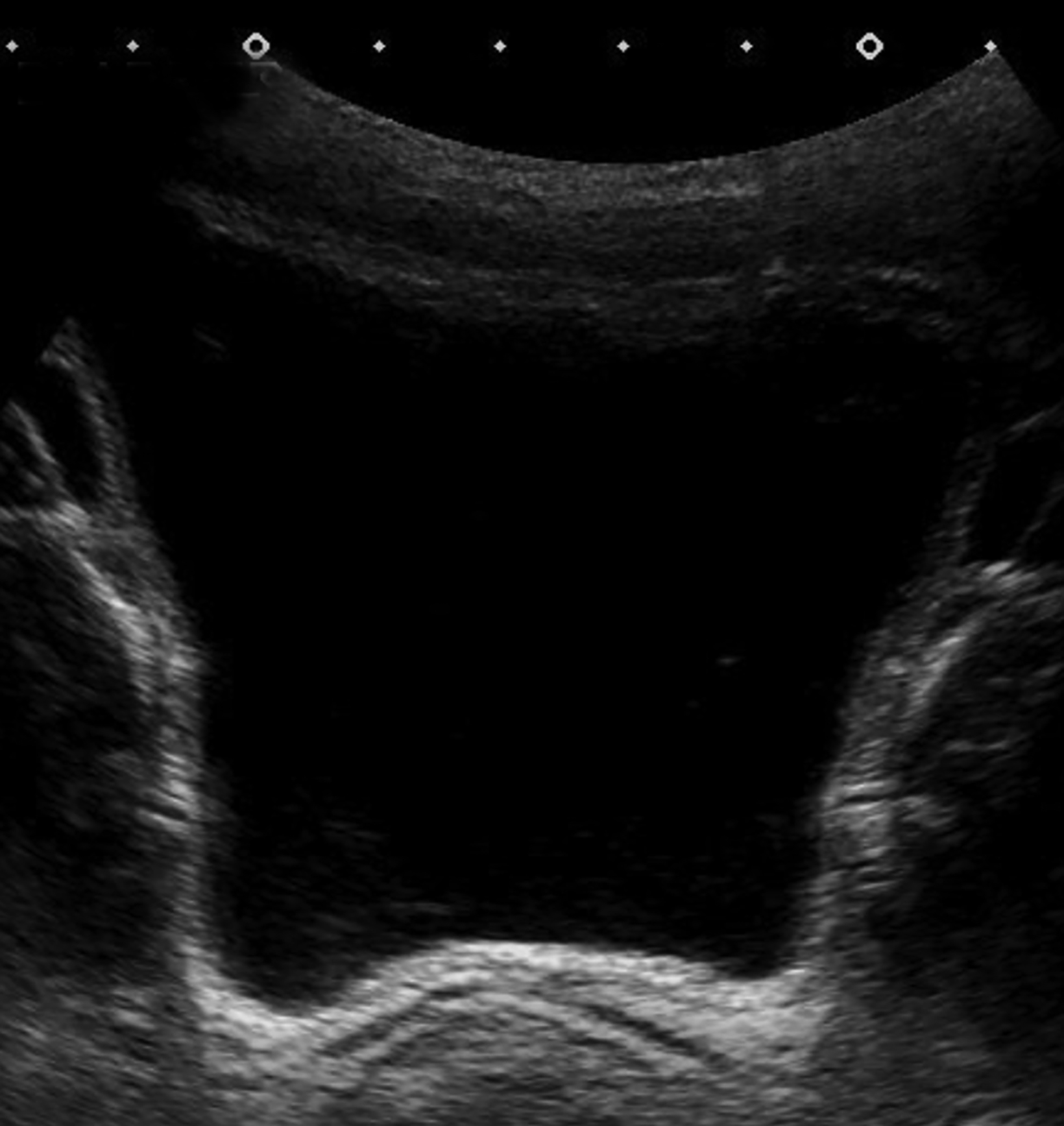
Bladder (Figure 1) and renal (not shown) ultrasound revealed a moderately distended urinary bladder with asymmetric, lobular, wall thickening at the fundus and left lateral wall. The urine contained moderate echogenic debris. There was no increased vascularity within the bladder wall. The kidneys were normal.
Diagnosis
Schistosoma bladder infection.
The differential diagnoses for microscopic hematuria and asymmetric bladder wall thickening in a child includes rhabdomyosarcoma and other bladder or urinary tract infections.
Discussion
Schistosomiasis is a parasitic disease caused by blood flukes. At least five known species infect humans, the three most common being Schistosoma haematobium, S japonicum, and S mansoni. The location of disease is determined by the species that infects the patient. For example, S haematobium usually infects the urinary system, while S japonicum and S mansoni migrate and infect areas in the gastrointestinal tract.1
The schistosoma blood flukes have a unique life cycle requiring two hosts to reproduce and survive. Blood fluke eggs are spread by feces or urine from mammals into a freshwater source. The eggs produce the miracidia form, which can infect freshwater snails.1 Once inside the snail, the miracidia undergo asexual reproduction into cercariae larvae.1 After the larvae leave the snail, the cercariae can directly penetrate mammal skin, then enter the bloodstream and migrate to the portal system of the liver, where they enter their sexual reproduction phase.1 At this point, S haematobium migrates to the venous plexus of the bladder, while S japonicum and mansoni migrate to the venules in the small and large intestine.2
Schistosomiasis is a tropical and subtropical disease affecting those who live in areas with poor sanitation. The different subtypes are endemic in different parts of the world: S mansoni is found throughout Africa, South America, and the Caribbean; S haematobium is found throughout Africa and the Middle East; and S Japonicum resides in Southeast Asia. Despite the worldwide distribution and transmission of schistosoma, 85% of cases of schistosomiasis are linked to Africa.2,3 The parasite is not native to the United States; patients diagnosed in the US will have a recent travel history, typically to an endemic region.
It is estimated that 230 to 250 million people contract schistosomiasis annually, with infection ultimately resulting in 280,000 deaths per year.1 Children are at increased risk for infection owing to higher rates of swimming and bathing in contaminated water sources. According to Colley, et al,4 the prevalence of infection is highest in those 10-14 years of age (~55%), with rates decreasing into adulthood.
Schistosoma can induce acute and chronic schistosomiasis. In both forms the symptoms and complications are caused by the host immune response provoking a granulomatous inflammatory reaction against the schistosome eggs.4 The acute form, also called Katayama fever, usually occurs weeks to months after the initial infection, when eggs are produced.
Presenting symptoms include fever, urticarial rash, myalgias, malaise, and abdominal pain lasting 2-10 weeks. Patients not treated then enter the chronic form of the disease where the immune response to the eggs is not as severe.
Patients infected chronically with one of the intestinal species of schistosoma may present with intermittent abdominal pain, diarrhea, and hematochezia. Periportal fibrosis and complications related to portal hypertension may occur if the immune response is not downregulated. Patients with a chronic infection caused by S haematobium most commonly present with hematuria, dysuria, urinary frequency, and suprapubic discomfort.
If the patient’s immune response is not downregulated, urinary tract fibrosis may occur, resulting in hydronephrosis and an increased risk of superimposed bacterial urinary tract infections. Early treatment is essential, as chronic bladder inflammation can lead to squamous metaplasia and squamous cell carcinoma.4
The diagnostic test of choice is microscopy for schistosome eggs in urine or stool. A bladder or rectal biopsy can be performed to examine for eggs, if not present in the urine or stool specimens. However, eggs are not produced for weeks to months following initial infection. Serology to detect antibodies against schistosoma can be used to screen for infection. However, patients from endemic areas are likely to have antibodies and thus, this test is not able to determine whether an infection is active.5
Praziquantel is the drug of choice for the treatment of schistosomiasis. It is thought praziquantel affects the parasite’s calcium channels. This causes adult worms to contract and detach from vein walls.5 The host immune system can recognize the worm antigens better after detachment.6 Patients have a good prognosis when treated early in the course of infection.7
The World Health Organization recommends annual or biannual prophylaxis with praziquantel for children and adults living in endemic areas to help suppress egg production.3,4 Other preventative measures include water sanitation programs and the elimination of intermediate snail hosts. A vaccine does not currently exist.4
Our patient was treated with a course of praziquantel. A bladder ultrasound performed after therapy showed resolution of the wall thickening.
Conclusion
Several species of schistosoma exist that can infect humans and cause differing complications. All species can cause the acute form of the disease. However, S mansoni and S japonicum usually cause complications in the gastrointestinal system, while S haematobium affects the urinary system. Schistosomiasis should be considered when patients residing in or having recently traveling to endemic regions have typical presentations.
Multiple modalities exist for diagnosis, including microscopy, biopsy, and serology. Bladder and kidney ultrasounds are essential to determine bladder damage caused by S haematobium and to rule out other causes of microscopic hematuria. Prognosis is good with early treatment, and praziquantel remains the drug of choice. Preventative efforts include mass chemoprophylaxis of endemic populations and water sanitation.
References
Citation
AA W, AJ T, D M, RB T. Schistosoma Bladder Infection. Appl Radiol. 2023; (3):50-52.
May 5, 2023