Samsung’s NeuroLogica receives FDA clearance for integrated ultrasound exam chair
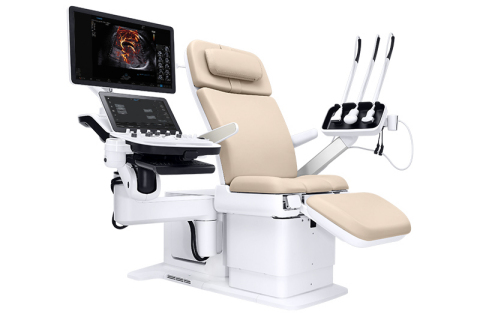 The Samsung Hera I10 ultrasound system from NeuroLogica was introduced at the 2019 annual conference of the Society of Diagnostic Medical Sonography, held last month in National Harbor, MD. This first-of-its-kind ultrasound system design, with the integration of an ultrasound examination chair, recently received clearance from the U.S. Food and Drug Administration (FDA).
The Samsung Hera I10 ultrasound system from NeuroLogica was introduced at the 2019 annual conference of the Society of Diagnostic Medical Sonography, held last month in National Harbor, MD. This first-of-its-kind ultrasound system design, with the integration of an ultrasound examination chair, recently received clearance from the U.S. Food and Drug Administration (FDA).
The integrated motorized chair “floating console” design enables clinicians to help patients safely and comfortably move into an optimal position for the exam, according to NeuroLogica. Its wide range of motion allows the system to adapt to the unique scanning position of each use to create an optimized skanning environment and to help reduce pain and strain of the sonographer.
The chair can support patient weight of up to 440 pounds. It can be adjusted from 18.9” to a maximum height of 38.6”. It also supports the majority of the transducer cable’s weight to reduce force on a scanner’s wrist by up to 70%.
The Samsung Hera I10 offers ShadowHDR™, which helps suppress shadows caused by fetal limbs or bone; LumiFlow™, which Provides dimensional visualization of blood flow that aids in quickly understanding vessel boundaries, and may provide additional spatial comprehension when documenting fetal and maternal hemodynamics; and MV-Flow™, which provides detailed visualization of microvascular perfusion into tissues and organs, for better visualization of fetal lung perfusion, ductus venosus, MCA, and adnexal low-flow hemodynamics.
