Sacrococcygeal Teratoma
Images
Case Summary
An infant with a prior history of urinary tract infections and negative renal ultrasound and voiding cystourethrogram presented for evaluation of sacral swelling.
Imaging Findings
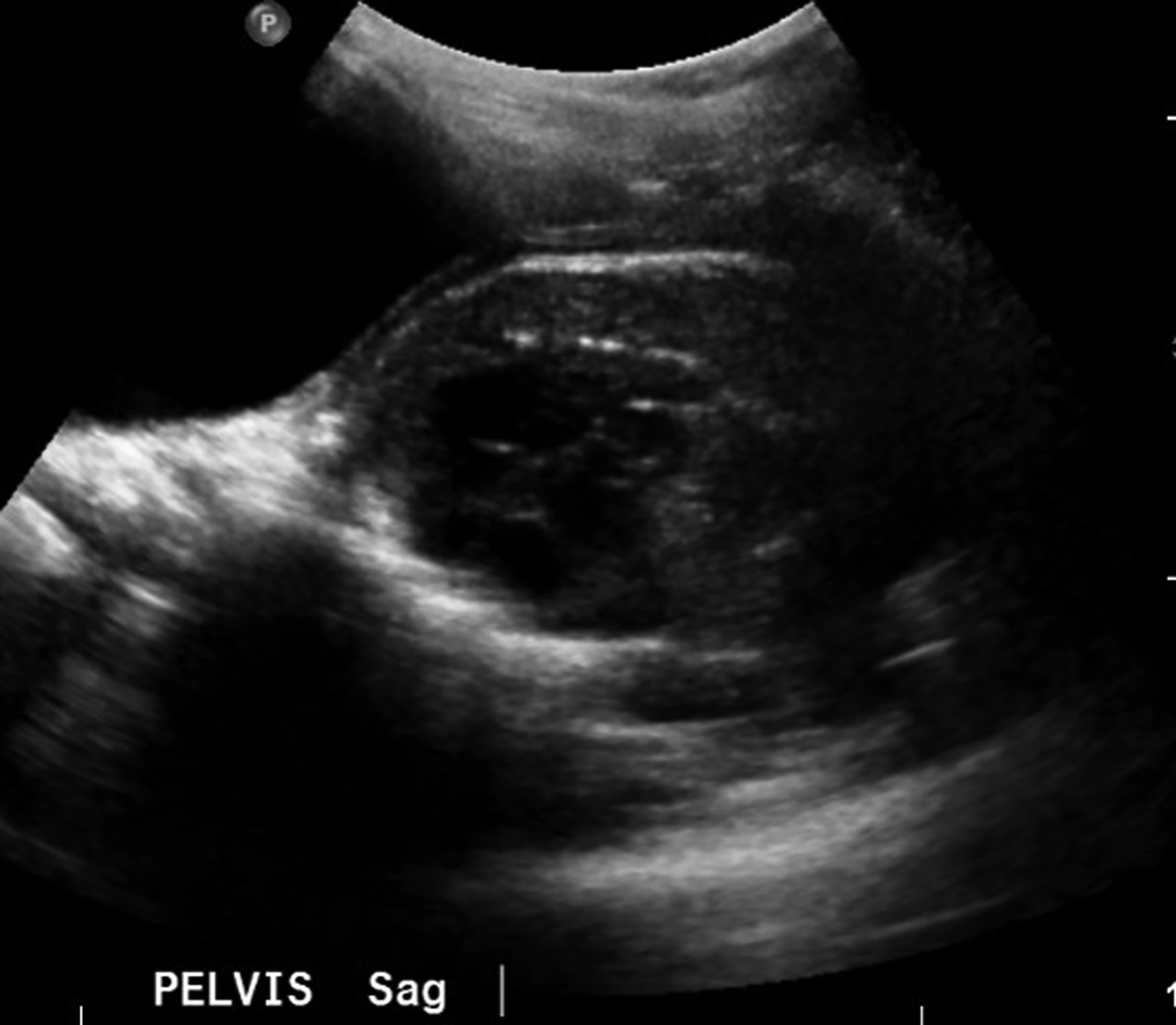
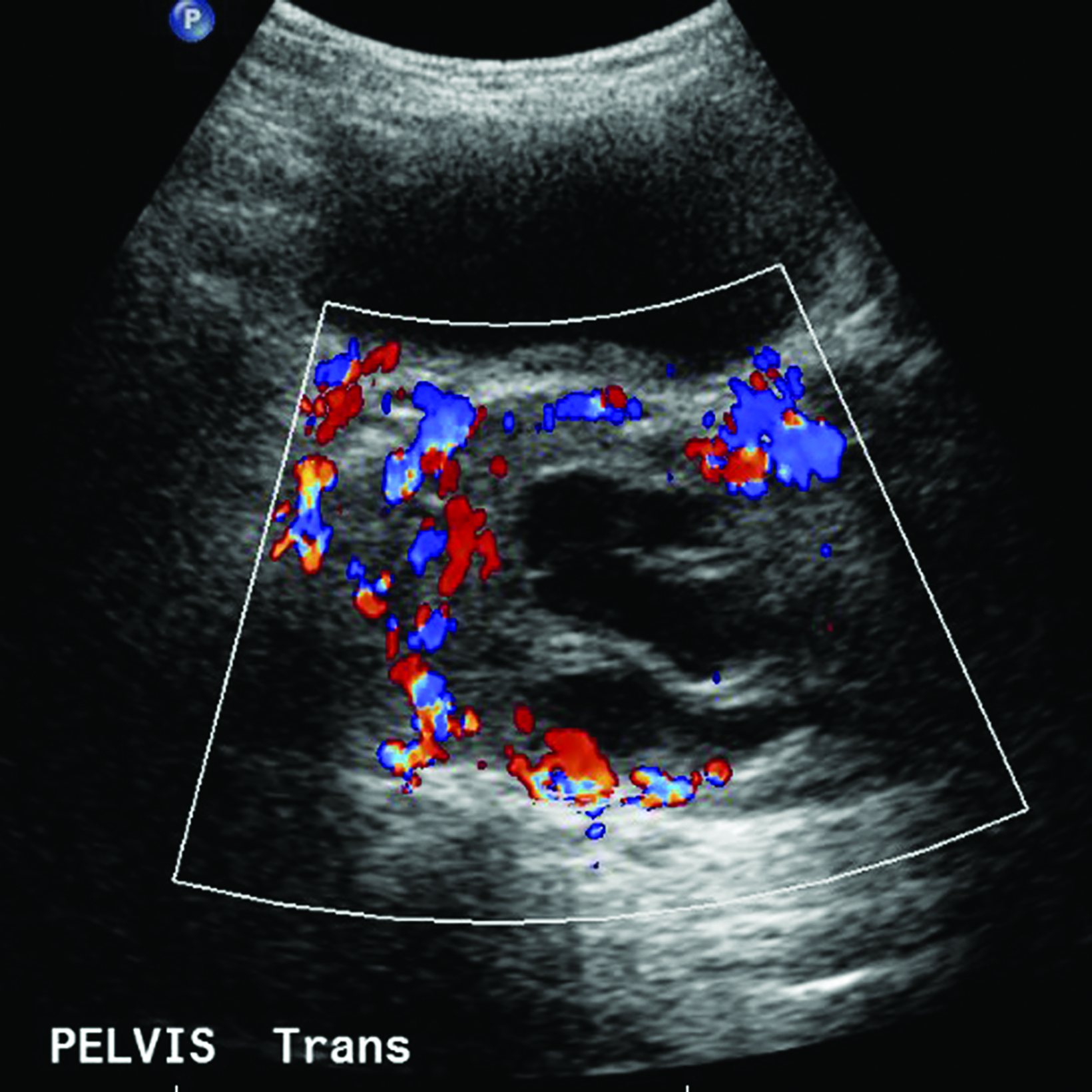
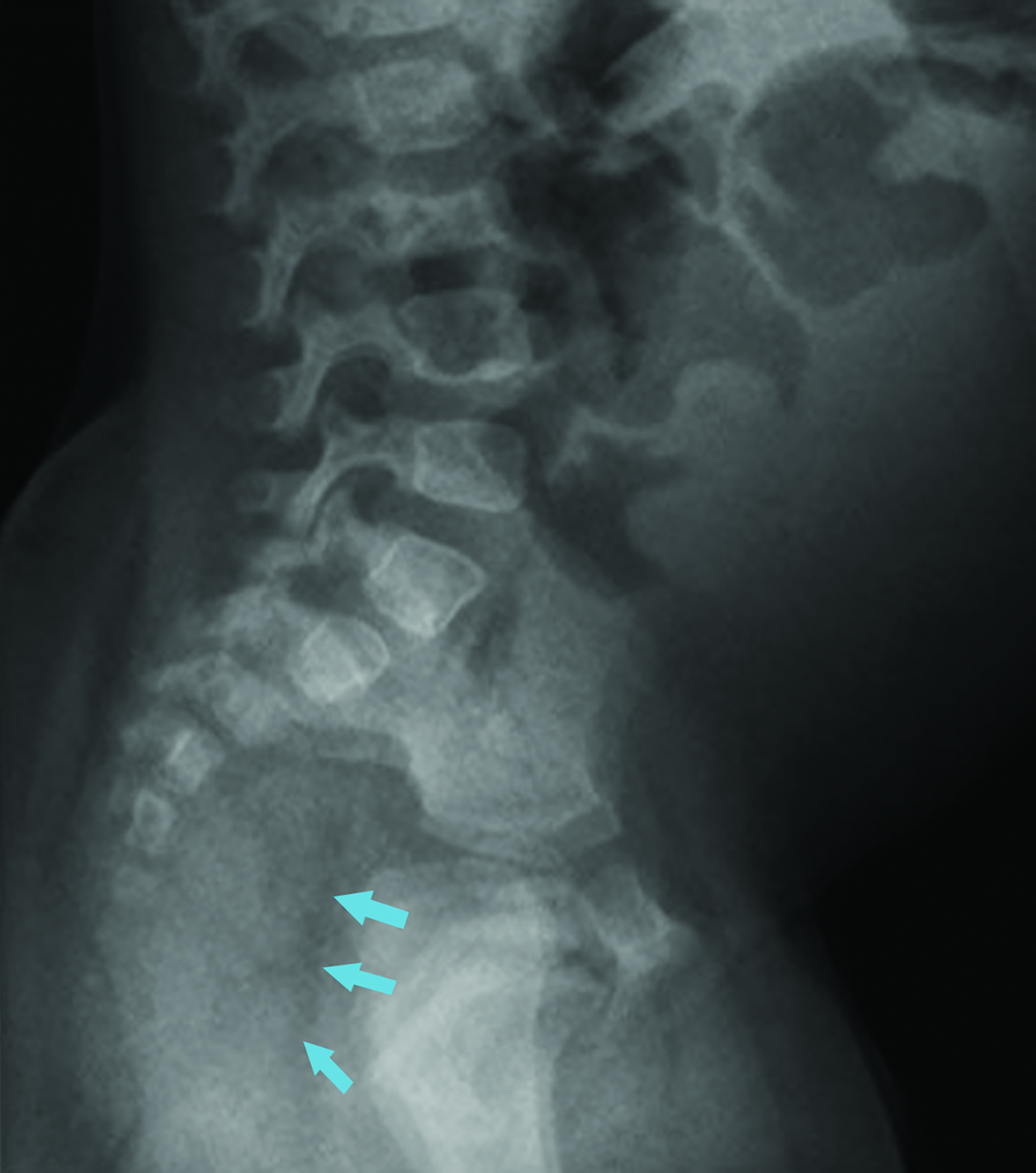
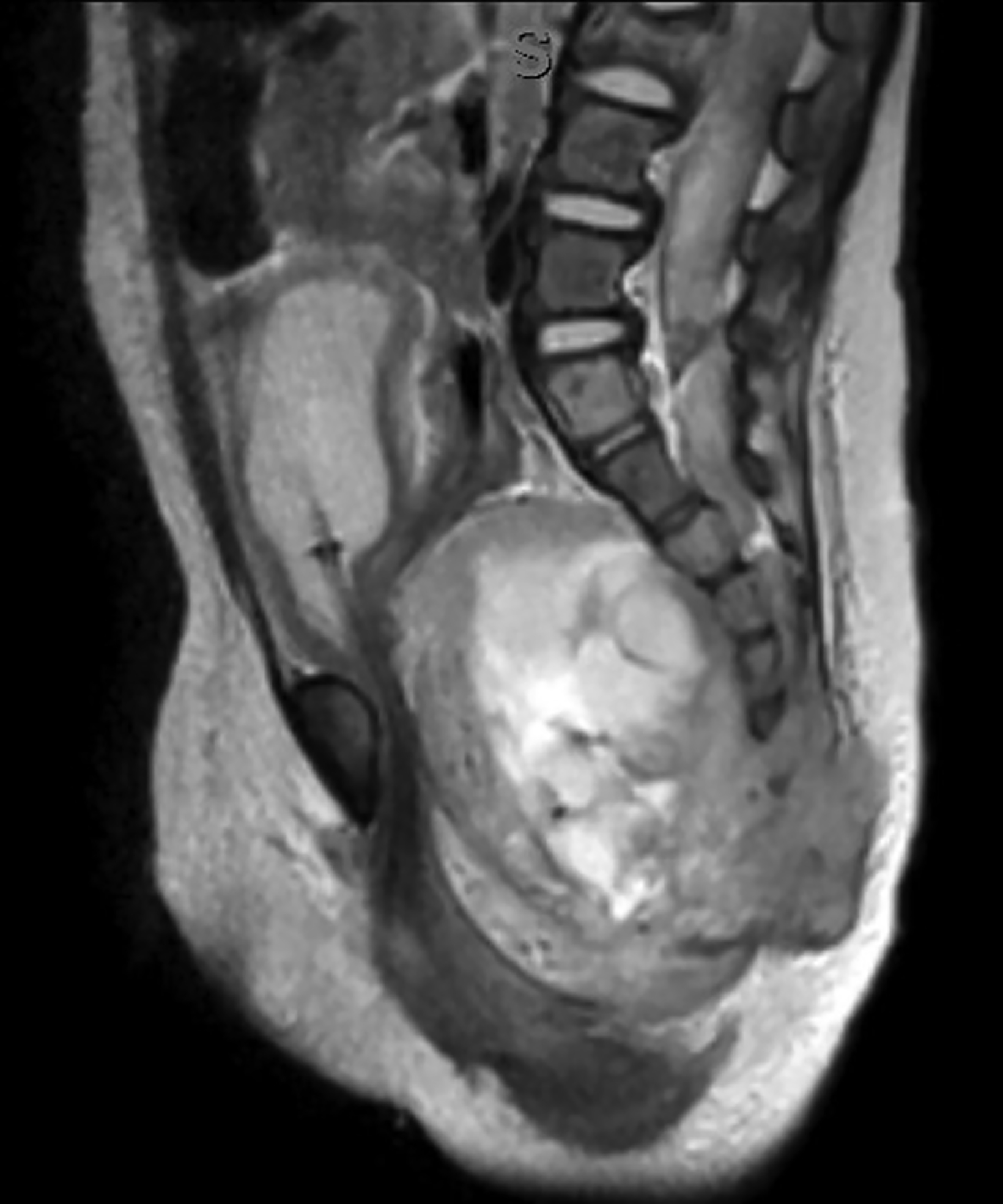
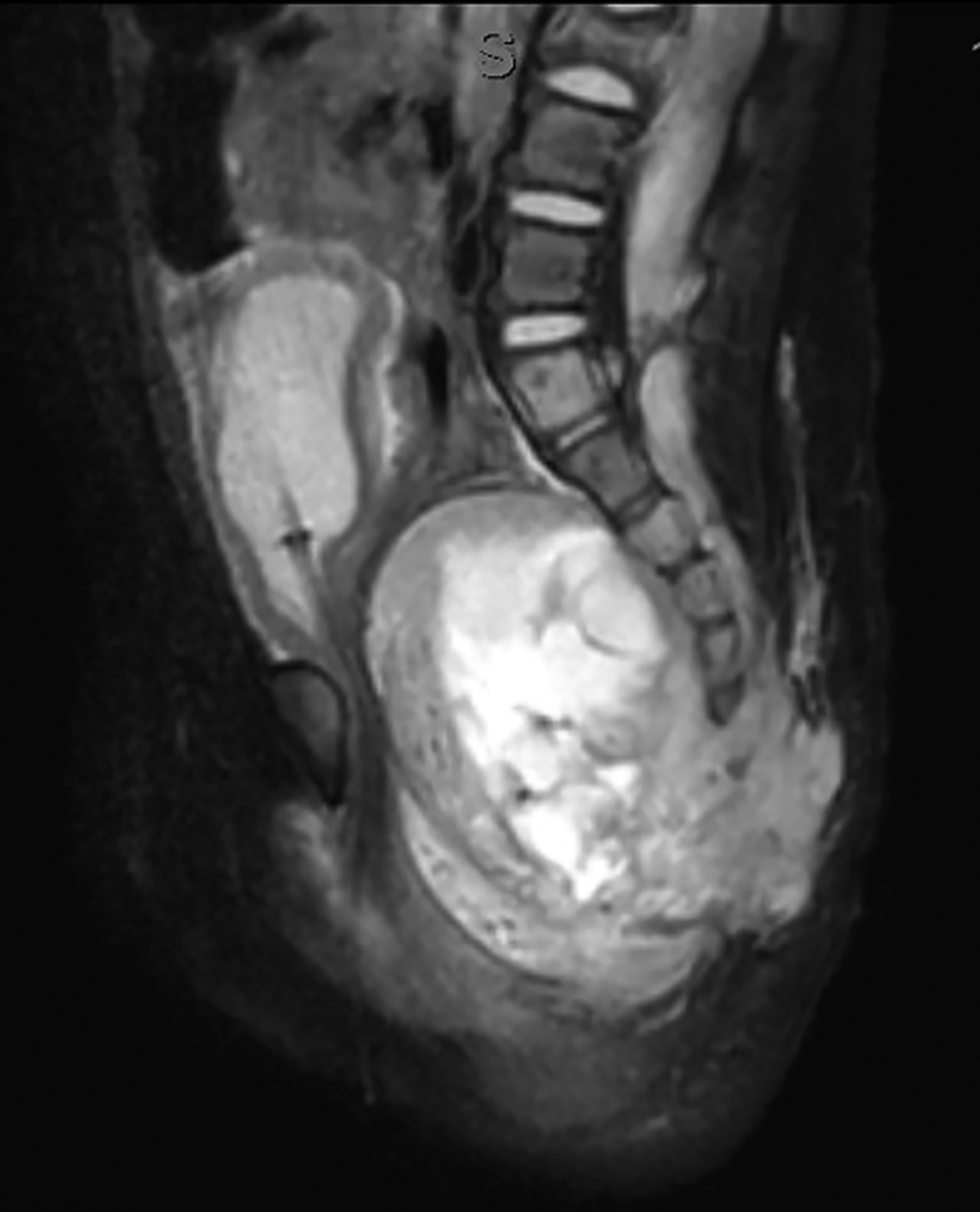
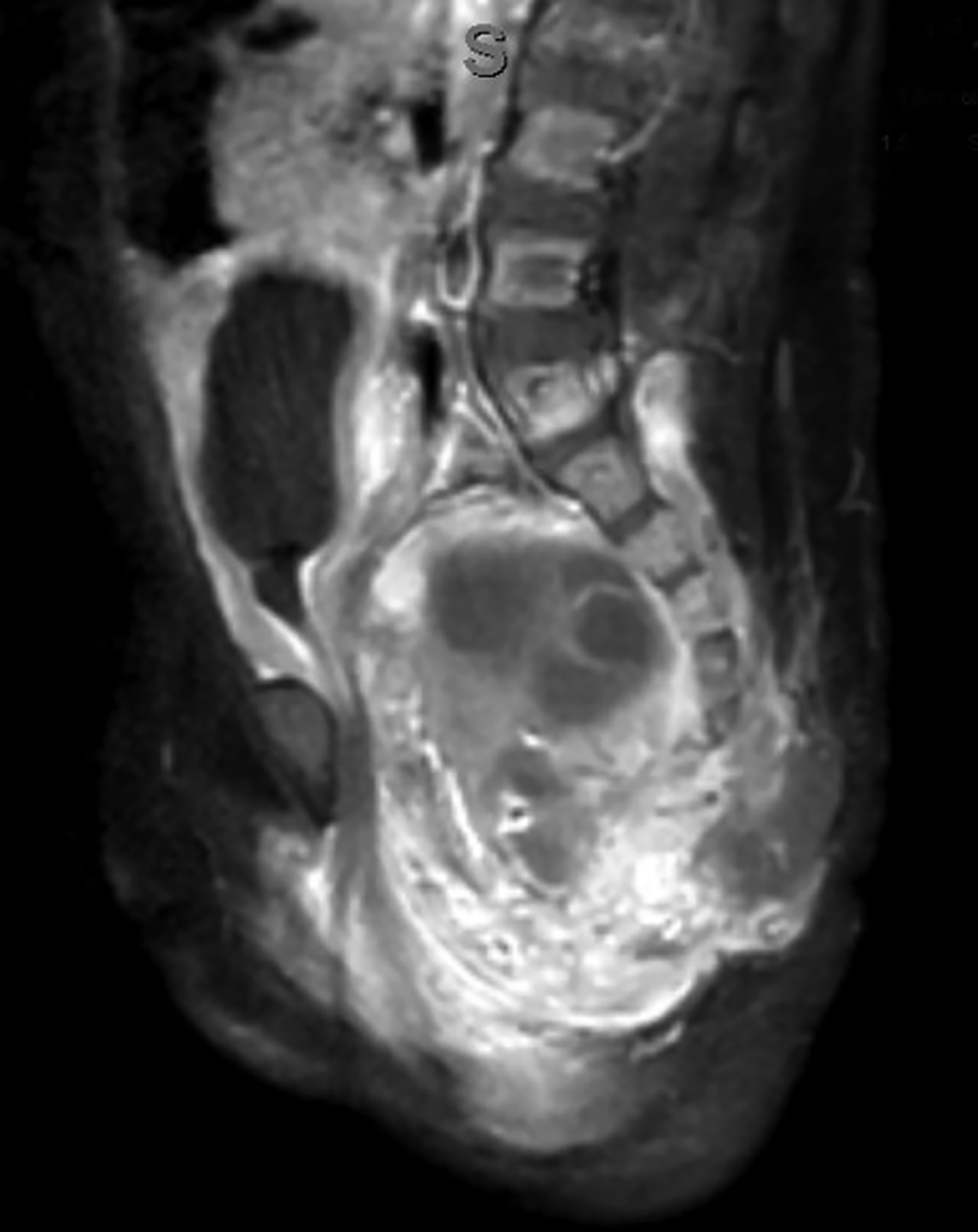
Ultrasonography over the sacrum (Figure 1) revealed a cystic/solid pre-sacral soft tissue mass. Lumbosacral spine radiograph demonstrated soft tissue density projecting over the sacrum and rectum with ill-defined, irregular cortices of the distal sacrum and coccyx (Figure 2). Pelvic contrast-enhanced magnetic resonance imaging (MRI) confirmed a 7 cm mass with a sacrococcygeal teratoma. It also showed spinal canal, sacral and lumbar vertebral-body involvement including L3 vertebral body height loss, (Figure 3).
Diagnosis
Sacrococcygeal teratoma (SCT).
The differential diagnosis includes SCT and congenital neural tube defects, such as an anterior sacral meningocele. In addition, one should consider benign lesions and malignant tumors. Benign lesions that could involve the pre-sacral region would include lymphatic malformations, dermoid cysts, and enteric cysts. Neoplastic pathologies including sacral chordomas, sacral schwannoma, neurofibroma, neuroblastomas, and rhabdomyosarcomas are considerations.
Discussion
A sacrococcygeal teratoma (SCT) is a germ cell tumor that is located close to the sacrum and coccyx. SCT arises from aberrant migration of primordial germ cells from the yolk sac to the gonads and accounts for approximately 3% of cancers in children <15 years of age.1 SCTs are the most common extragonadal germ cell tumor (GCT) in young children. SCT is more common in girls than boys [4:1]. Most SCTs are benign but they may grow to a large size. If rapid tumor growth occurs in utero the associated high blood flow can result in heart failure and hydrops. Although the differential diagnosis for a sacral mass can include a neural tube defect, such as a myelocystocele or a myelomeningocele, SCTs are more likely to be presacral rather than dorsal to the sacrum, leading to mass effect such as bladder outlet obstruction, hydronephrosis, and rectal stenosis or atresia.2
GCTs can be divided into three main subtypes: mature, immature, and malignant. Malignant teratomas can be challenging to accurately diagnose as malignant components may be missed on histologic examination. Therefore, screening with beta-human chorionic gonadotropin or alpha-fetoprotein is important to assess for malignant components.2 Specifically, SCTs are often described using the Altman Classification, which morphologically categorizes according to the extent to which SCT is external or internal, with Type 1 being primarily external and Type 4 being primarily internal. Type 1 and Type 2 SCTs are often detected with prenatal ultrasound, as the external component is more easily observed; Type 4 SCTs typically do not present until infancy and early childhood. Type 4 SCTs are more commonly associated with malignant components.3
Sacrococcygeal teratomas frequently present in utero or early infancy and may be asymptomatic or can present with weakness, pain, paralysis, and/or symptoms related to bladder or rectal obstruction.4 Approximately 32% of SCTs are diagnosed postnatally during the initial newborn assessment that occurs within the first 24 hours following birth.5 However, SCTs are often diagnosed in utero by obstetrical ultrasound or fetal MRI.5 Once identified, serial ultrasounds are performed with the primary goals of (1) identifying fetuses with a high risk of hydrops and (2) intervening as necessary.
Ultrasonography of mature SCTs can show anechoic regions, indicating cystic components of the tumor.6 Ultrasonography, including color Doppler, of these tumors can also assess for tumor size, high-output cardiac states, and vascular steals, which are associated with increased risk for the progression to hydrops.7 Prenatal monitoring also allows for the identification of positive prognostic factors, such as cystic tumor type and minimal or no abnormal vascularity, and poor prognostic factors, including hydrops, large size, solid tumor with hypervascularity, and immaturity.5,8
The treatment of SCTs diagnosed in utero often focuses on managing the tumor’s effect on the cardiovascular system to decrease the risk of the development of hydrops and allow the fetus to mature appropriately. Fetal surgery, via laser or radiofrequency ablation, and minimally invasive surgery, may be indicated in certain high-risk cases of SCTs with poor prognostic factors for survival such as high-output cardiac failure and intra-lesional hemorrhage.9,10
Postnatally, SCTs are evaluated with MRIs, the diagnostic procedure of choice. Radiography may demonstrate a calcified mass from the lower pelvic region and the impact of the mass effect and compression of adjacent structure such as colonic displacement, ureteric displacement, and dilatation, intraspinal extension, and metastasis.6 MRI can show the full extent of the tumor and complications resulting from it, including colonic displacement, ureteric dilatation, intraspinal extension, and metastases.5 Treatment often includes surgical resection. Complete removal of SCTs must include removal of the entire coccyx, but it may be difficult to achieve complete resection of SCTs because they lack capsular or pseudo-capsular components.
Treatment after delivery of a neonate with SCT is surgical resection. In some cases, multiple procedures may also be necessary to ensure complete resection of the tumor, especially in cases where malignant components of the tumor are detected.11
Following surgery, benign SCTs are often managed with observation. In contrast, malignant and/or metastatic SCTs often require adjuvant or neoadjuvant chemotherapy. Recurrence of SCTs can occur either locally or at distant sites, and management includes additional surgeries or chemotherapy depending on the pathology of the tumor. Survival rates of SCTs also differ based on histopathology. Mature, benign SCTs have a survival rate of approximately 98% compared to a 60% survival rate for immature, malignant SCTs.5
Conclusion
Sacrococcygeal teratomas are commonly diagnosed in utero using prenatal ultrasonography. High-risk lesions may be monitored with serial ultrasound so that surgical intervention can be performed in fetuses at high risk for hydrops and fetal demise. In neonates, SCTs often present with symptoms of weakness, pain, paralysis, and urologic and/or anorectal dysfunction. Treatment of SCTs typically involves postnatal surgical resection and chemotherapy in those with malignant changes.
References
- Yadav DK, Acharya SK, Bagga D, Jain V, Dhua A, Goel P. Sacrococcygeal teratoma: clinical characteristics, management, and long-term outcomes in a prospective study from a tertiary care center. J Indian Assoc Padiatr Surg. 2020; 25:15-21
- Yu JA, Sohaey R, Kennedy AM, Selden NR. Terminal myelocystocele and sacrococcygeal teratoma: a comparison of fetal ultrasound presentation and perinatal risk. AJNR Am J Neuroradiol. 2007; 28(6):1058-1060. https://doi.org/10.3174/ajnr.A0502.
- Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg. 1974; 9(3):389-398. doi: 10.1016/s0022-3468(74)80297-6.
- Mahour GH. Sacrococcygeal teratomas. CA Cancer J Clin. 1988; 38(6):362-367. https://doi.org/10.3322/canjclin.38.6.362.
- Ayed A, Tonks AM, Lander A, Kilby MD. A review of pregnancies complicated by congenital sacrococcygeal teratoma in the West Midlands region over an 18-year period: population-based, cohort study. Prenat Diagn. 2015; 35(11):1037-1047. doi: 10.1002/pd.4641.
- Smith D, Weerakkody Y. Sacrococcygeal teratoma. Radiopaedia. https://radiopaedia.org/articles/sacrococcygealteratoma?lang=us. Accessed May 15, 2020.
- Flake AW. Fetal sacrococcygeal teratoma. Semin Pediatr Surg. 1993; 2(2):113-120. https://pubmed.ncbi.nlm.nih.gov/8062028/. Accessed May 3, 2020.
- Benachi A, Durin L, Vasseur Maurer S, et al. Prenatally diagnosed sacrococcygeal teratoma: a prognostic classification. J Pediatr Surg. 2006; 41(9):1517-1521. doi: 10.1016/j.jpedsurg.2006.05.009.
- Lee MY, Won HS, Hyun MK, et al. Perinatal outcome of sacrococcygeal teratoma. Prenat Diagn. 2011; 31(13):1217-1221. doi: 10.1002/pd.2865.
- Van Mieghem T, Al-Ibrahim A, Deprest J, et al. Minimally invasive therapy for fetal sacrococcygeal teratoma: case series and systematic review of the literature. Ultrasound Obstet Gynecol. 2014; 43(6):611-609. doi: 10.1002/uog.13315.
- Calaminus G, Schneider DT, Bökkerink JP, et al. Prognostic value of tumor size, metastases, extension into bone, and increased tumor marker in children with malignant sacrococcygeal germ cell tumors: a prospective evaluation of 71 patients treated in the German cooperative protocols Maligne Keimzelltumoren (MAKEI) 83/86 and MAKEI 89. J Clin Oncol. 2003; 21(5):781-786. doi: 10.1200/JCO.2003.03.125.
References
Citation
JJ N, RB T, CM S, AJ T. Sacrococcygeal Teratoma. Appl Radiol. 2022;(4):59-62.
July 14, 2022