Renal Artery Pseudoaneurysm
Images
Case Summary
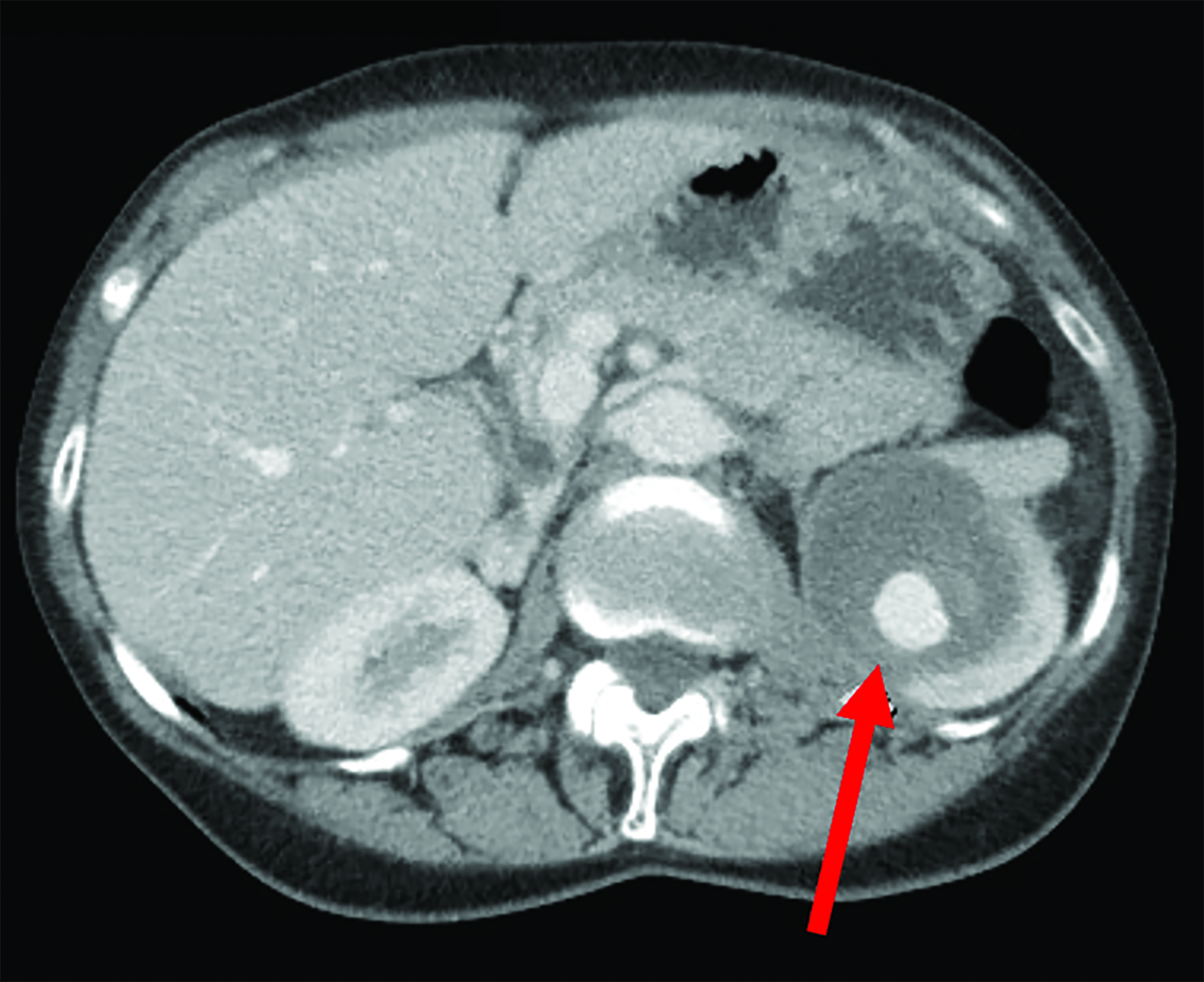
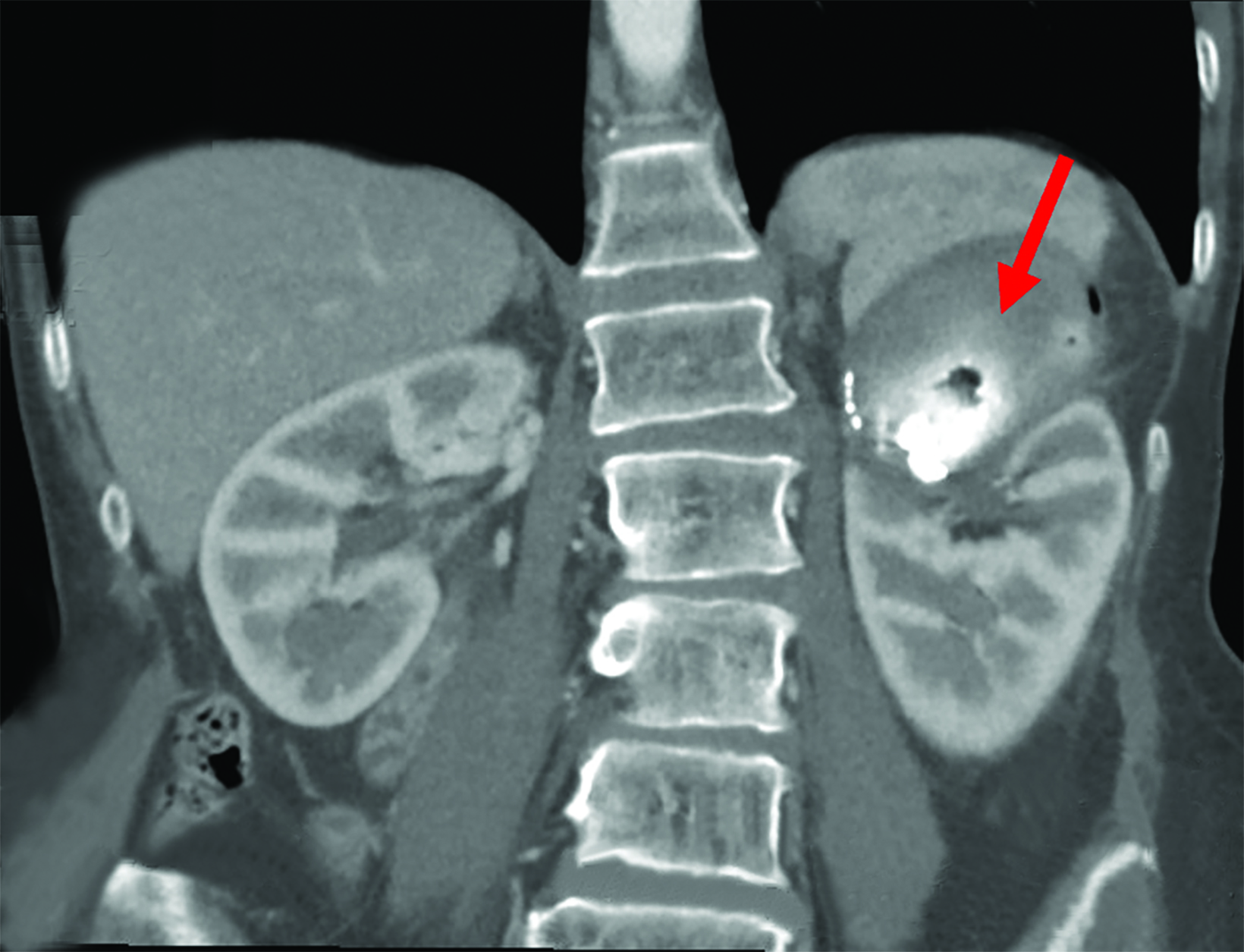
An adult patient underwent laparoscopic partial nephrectomy of a 3.3 cm mass in the upper pole of the left kidney (Figure 1). The patient developed left abdominal pain 4 weeks later and an outpatient ultrasound (US) demonstrated a large left renal artery pseudoaneurysm. There was no hematuria.
Imaging Findings
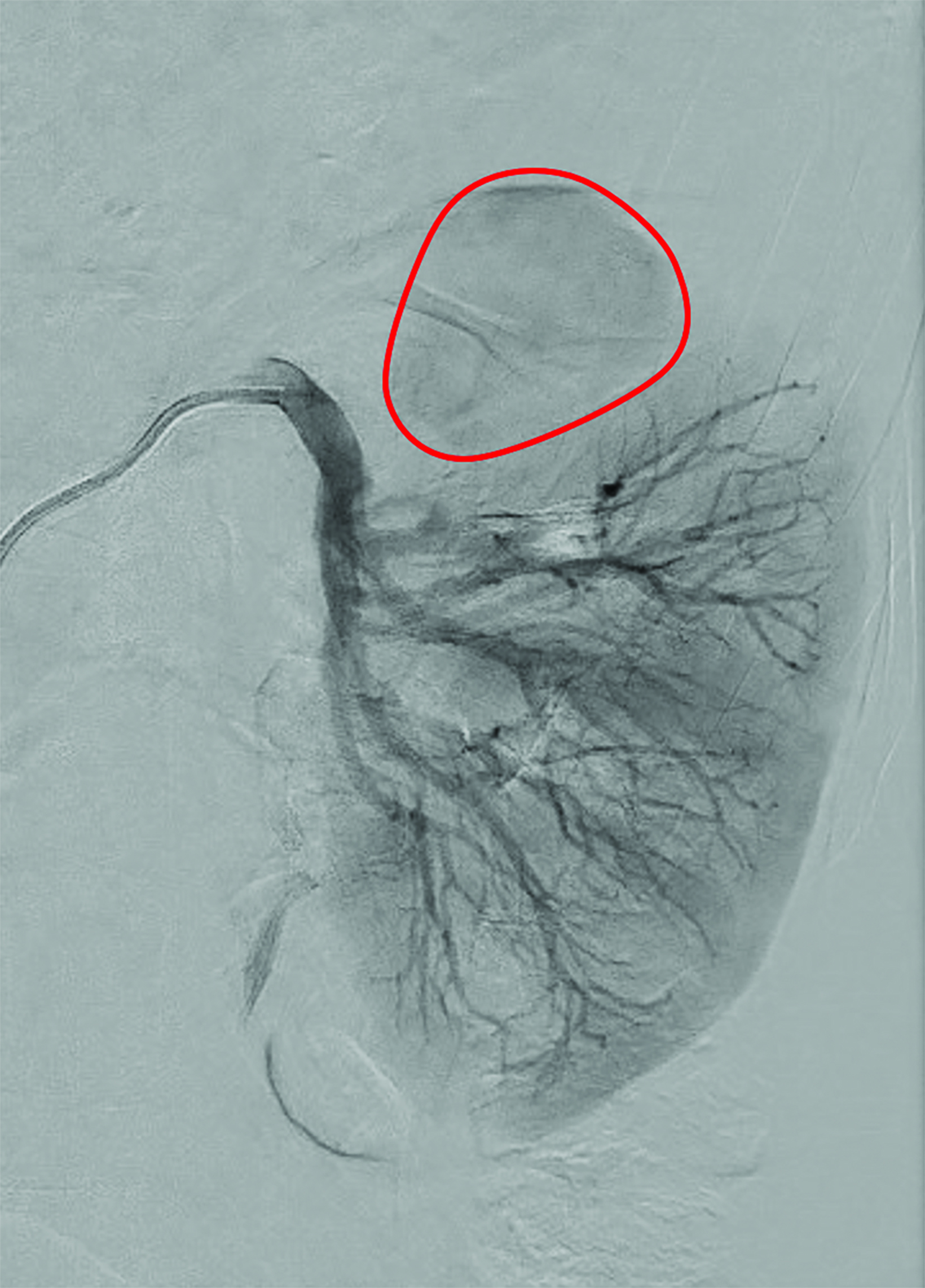
Contrast-enhanced computed tomography (CT) study in 3 phases demonstrated a 7.5 x 5.5 cm pseudoaneurysm with an enhancing nidus (4.6 x 2.5cm) in the upper pole of the left kidney (Figure 1). Ultrasound-guided thrombin injection was performed, and the pseudoaneurysm sac seen to thrombose on intraprocedural ultrasound. The pseudoaneurysm recanalized 24 hours later. Transfemoral left renal angiogram identified the pseudoaneurysm (Figure 1). Microcatheter access of the small branch to the pseudoaneurysm for sub-selective embolization was ultimately unsuccessful due to the short, tortuous nature of the vessel.
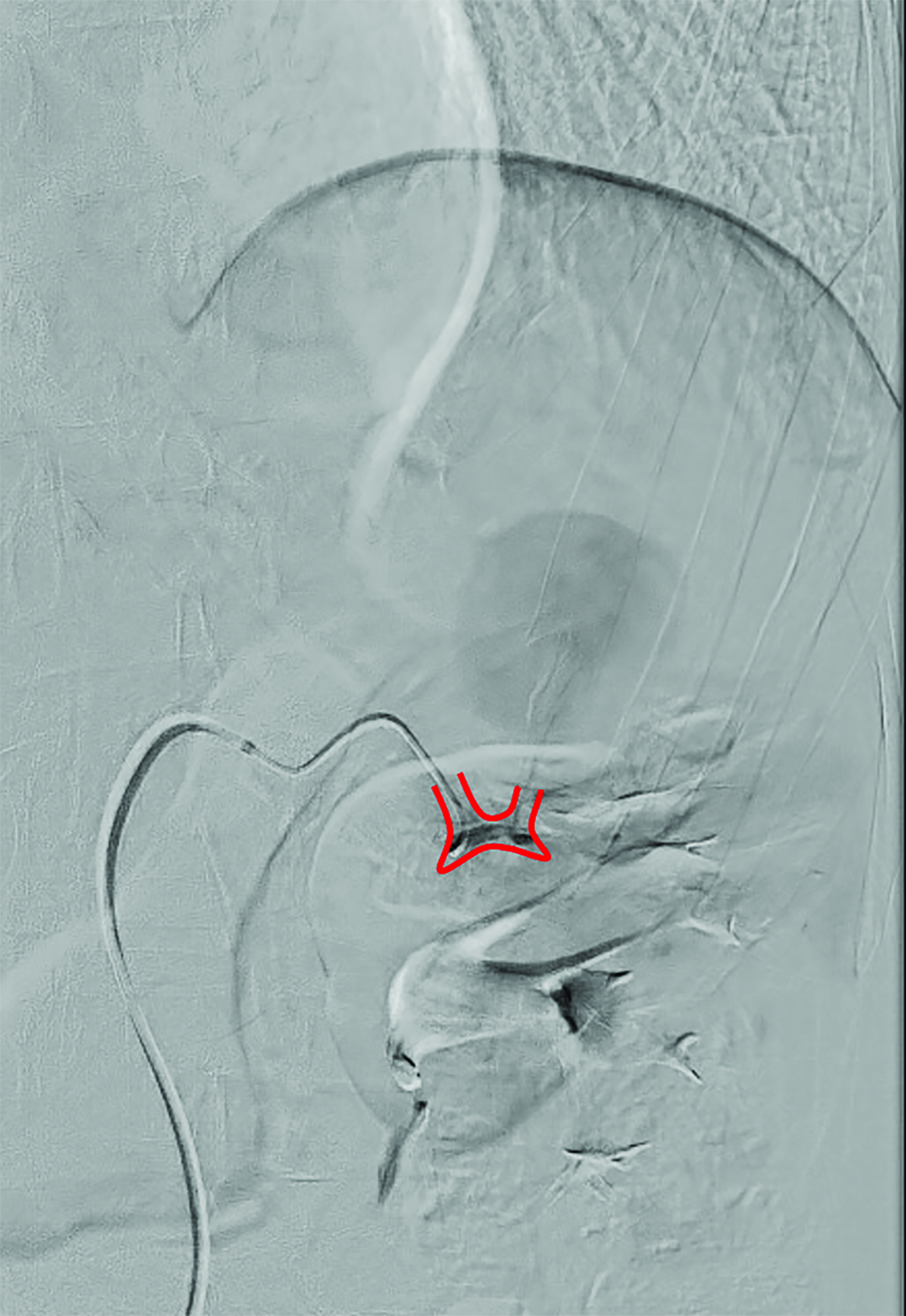
The pseudoaneurysm sac was subsequently accessed percutaneously under ultrasound guidance during the same treatment session. The neck of the pseudoaneurysm was embolized with 3 cc of a mixture of 2 cc of cyanoacrylate glue diluted in 5 cc of lipiodol. A Gelfoam slurry was also injected into the aneurysm sac to further facilitate thrombus formation once the neck had been embolized.
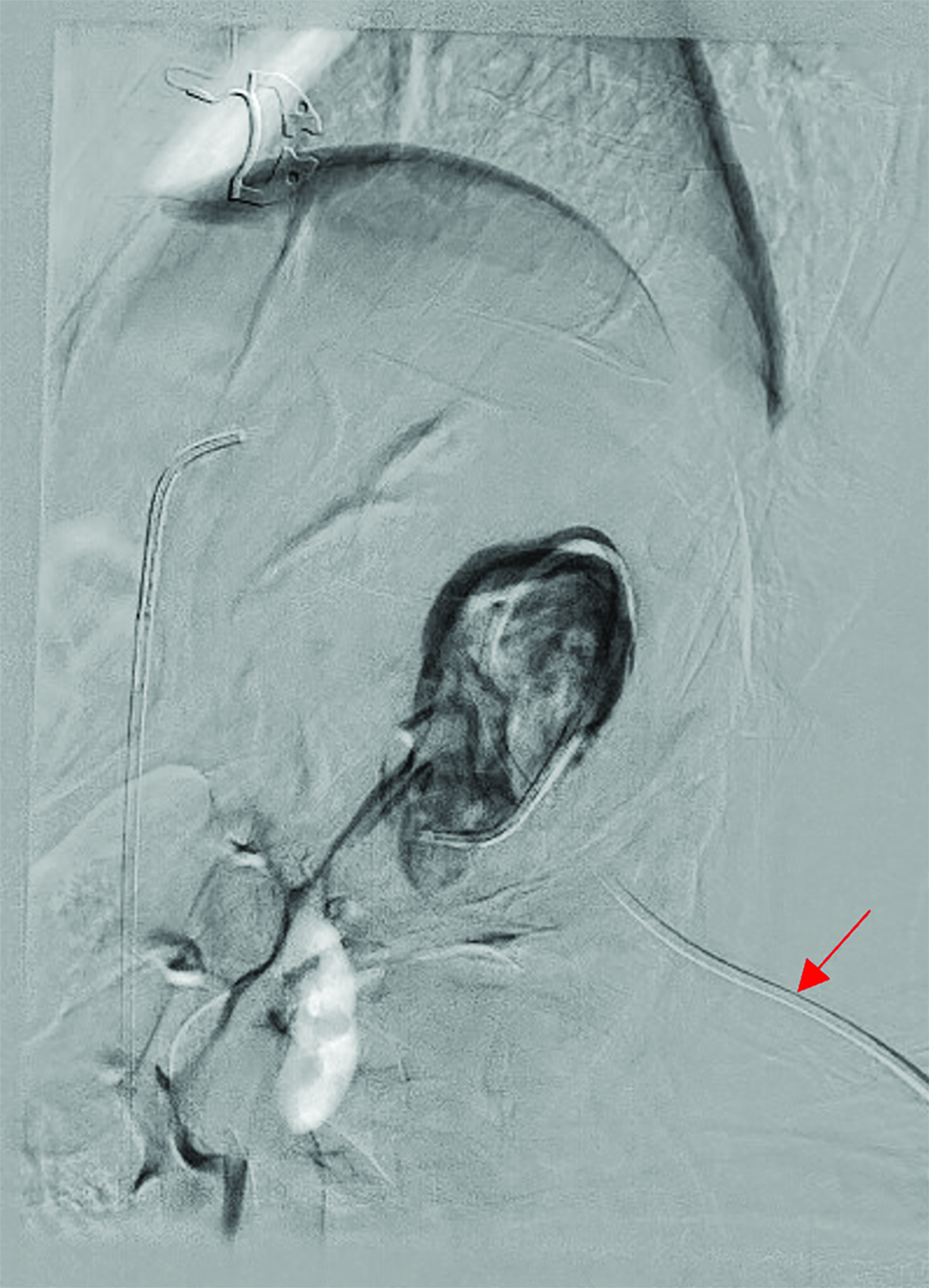
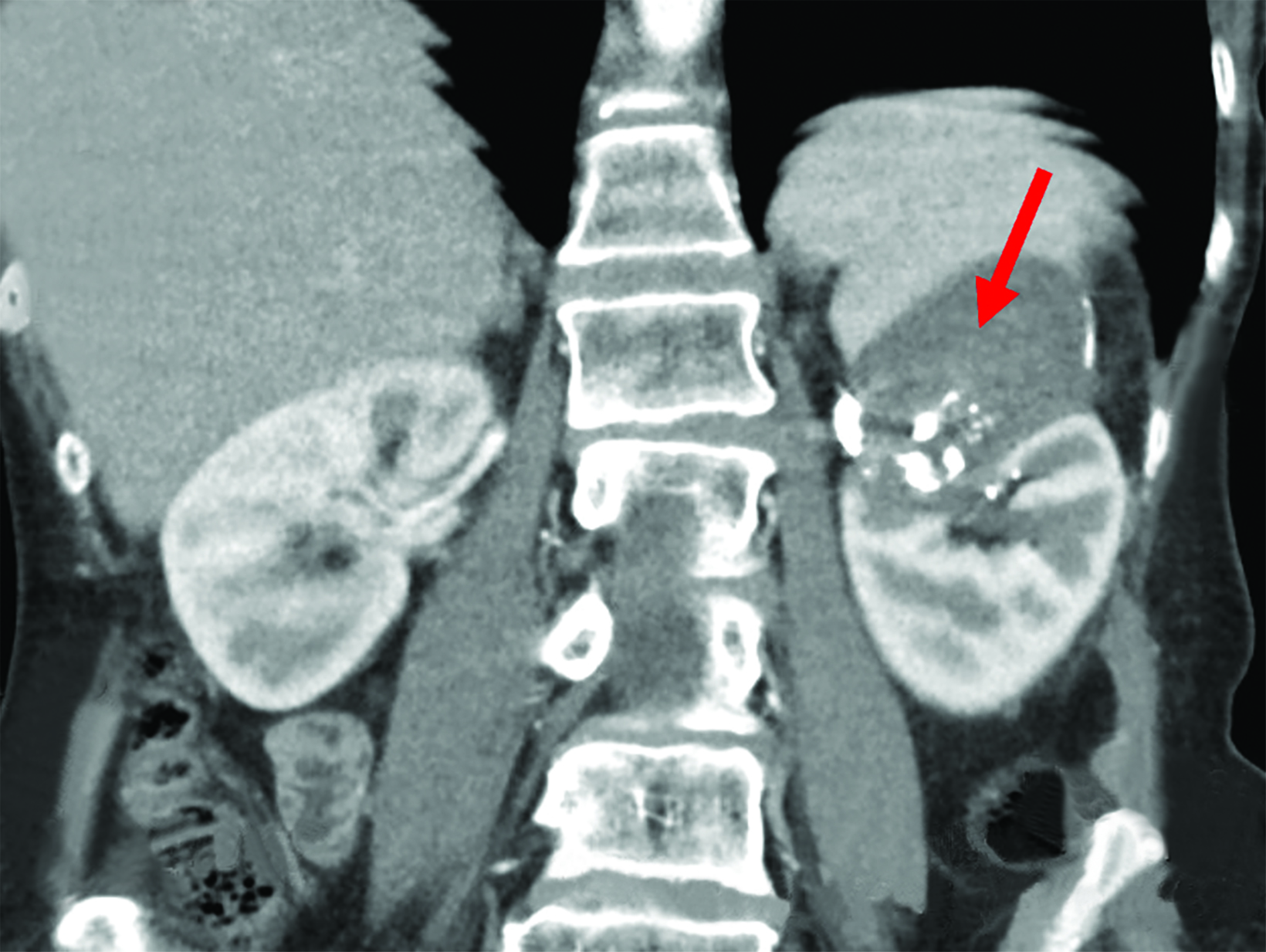
Follow-up CT scans at 24 hours and 2 months (Figure 2) demonstrated a completely thrombosed pseudoaneurysm.
Diagnosis
Renal artery pseudoaneurysm. Differential diagnosis on CT includes hematoma, abscess, and seroma.
Discussion
Renal artery pseudoaneurysm is a rare but life-threatening complication of partial nephrectomy, with a reported incidence reaching 18%.1Asymptomatic and small pseudoaneurysms can be observed and may spontaneously resolve without intervention.1 The mortality rate of a ruptured visceral pseudoaneurysm approaches 100% if unaddressed; the current standard of care is to treat if symptomatic.2
Major treatment modalities consist of endovascular and surgical approaches; US-guided compression is not applicable owing to the renal artery’s deep location. Endovascular repair entails occlusion of the pseudoaneurysm sac with coils or liquid embolic material; eg, Gelfoam, N-butyl cyanoacrylate, and is preferred over open surgical repair owing to its lower morbidity, mortality, and shorter postoperative hospital stay.
Surgery maintains a role as definitive treatment if endovascular repair fails, which occurs in up to 17.3% of cases.2 Renovascular tortuosity a significant predictor of initial treatment failure, according to two retrospective reviews examining failure of renal artery embolization of pseudoaneurysms and other causes of gross hematuria post-percutaneous nephrolithotomy.3,4
Ultrasound-guided direct embolization of renal artery pseudoaneurysms has been reported previously in the context of percutaneous nephrolithotomy, renal biopsy, and penetrating trauma;5,6 to urgently manage intraoperative bleeding;7 and for pseudoaneurysms of other sites such as hepatic arteries, including in emergency settings.6,8
One case series of 40 patients used an 18-gauge needle under duplex US to inject Gelfoam followed by N-butyl cyanoacrylate with complete technical success and resolution of hematuria.5 Others have used duplex US, 14-18 gauge needles, and Gelfoam only, finding good success with any narrow-necked pseudoaneurysm larger than 4 cm. Direct embolization has even demonstrated utility in cases where the feeding artery could not be identified.6 Hence, it is an emerging technique for rapidly and effectively managing renal pseudoaneurysms with minimal adverse effects.
Direct percutaneous embolization of the pseudoaneurysm sac is of particular benefit in patients with difficult anatomy, reducing the risks associated with open surgery (blood loss, general anesthesia, prolonged recovery time). It can also be attempted immediately after failure of initial endovascular repair, thus avoiding treatment delays associated with rescheduling for additional attempts. Used as an initial management strategy, we hypothesize that direct percutaneous embolization could even reduce the need for radiographic contrast media, providing an advantage in the care of the renally impaired.
Conclusion
We describe a rapid and effective alternative method of percutaneously accessing a pseudoaneurysm sac for use of liquid agent for embolization. It is especially applicable to patients with tortuous anatomy and for same-session repair. Remaining questions include the optimal relationship between needle gauge, embolic material, and pseudoaneurysm dimensions and properties.
A head-to-head comparison between endovascular embolization and direct embolization may be warranted to update current standards of care.
References
Citation
A W, M A, S A. Renal Artery Pseudoaneurysm. Appl Radiol. 2023;(2):39-41.
March 7, 2023