Removal of Subdermal Contraceptive Device
Images
Case Summary
An adult with an etonogestrel 68 mg subdermal implant (Nexplanon) placed in the left arm three years previous presented for removal of the implant due to irregular menstrual cycles. On physical examination, the device was difficult to palpate and likely embedded in the fascia or muscle wall.
Imaging Findings
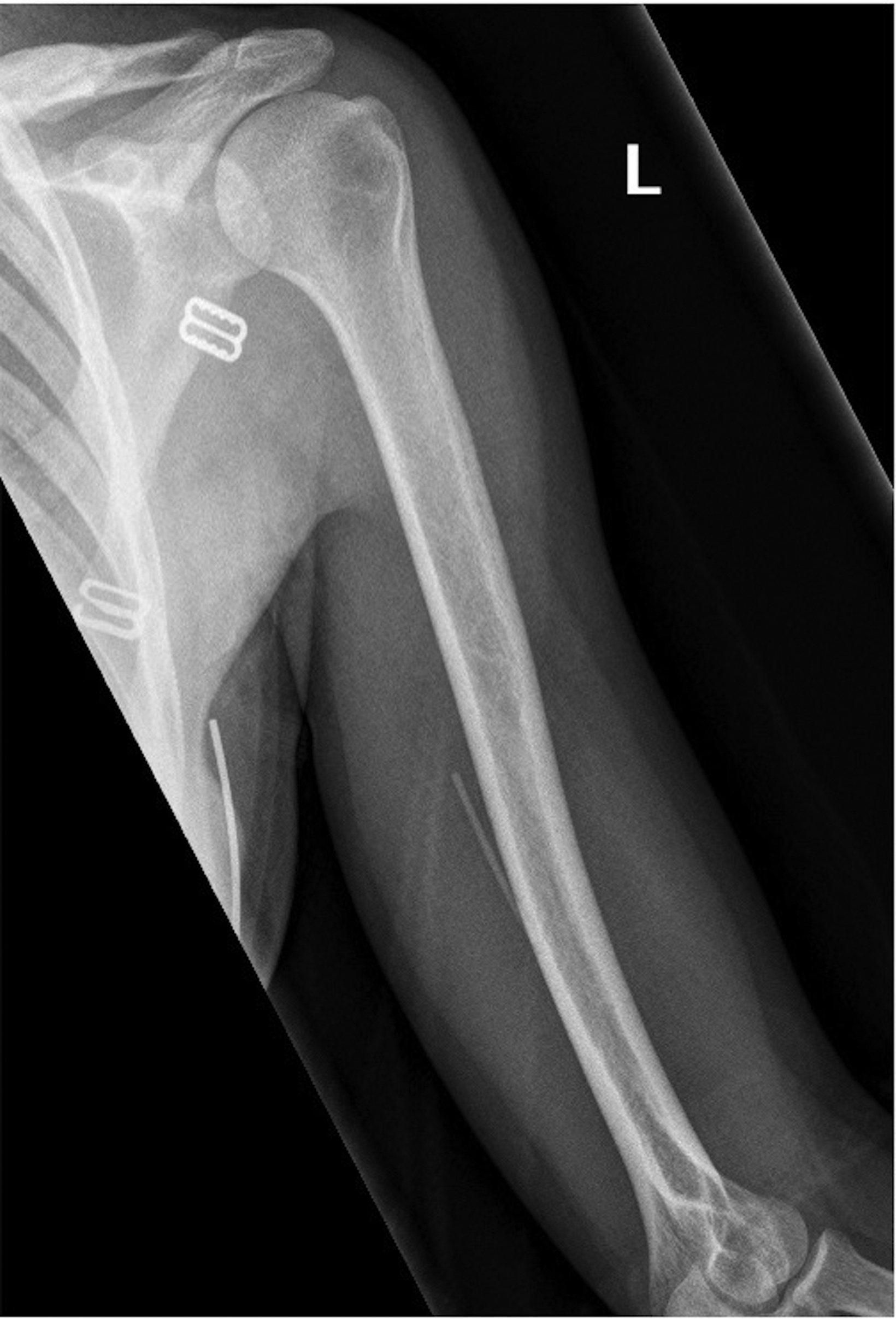
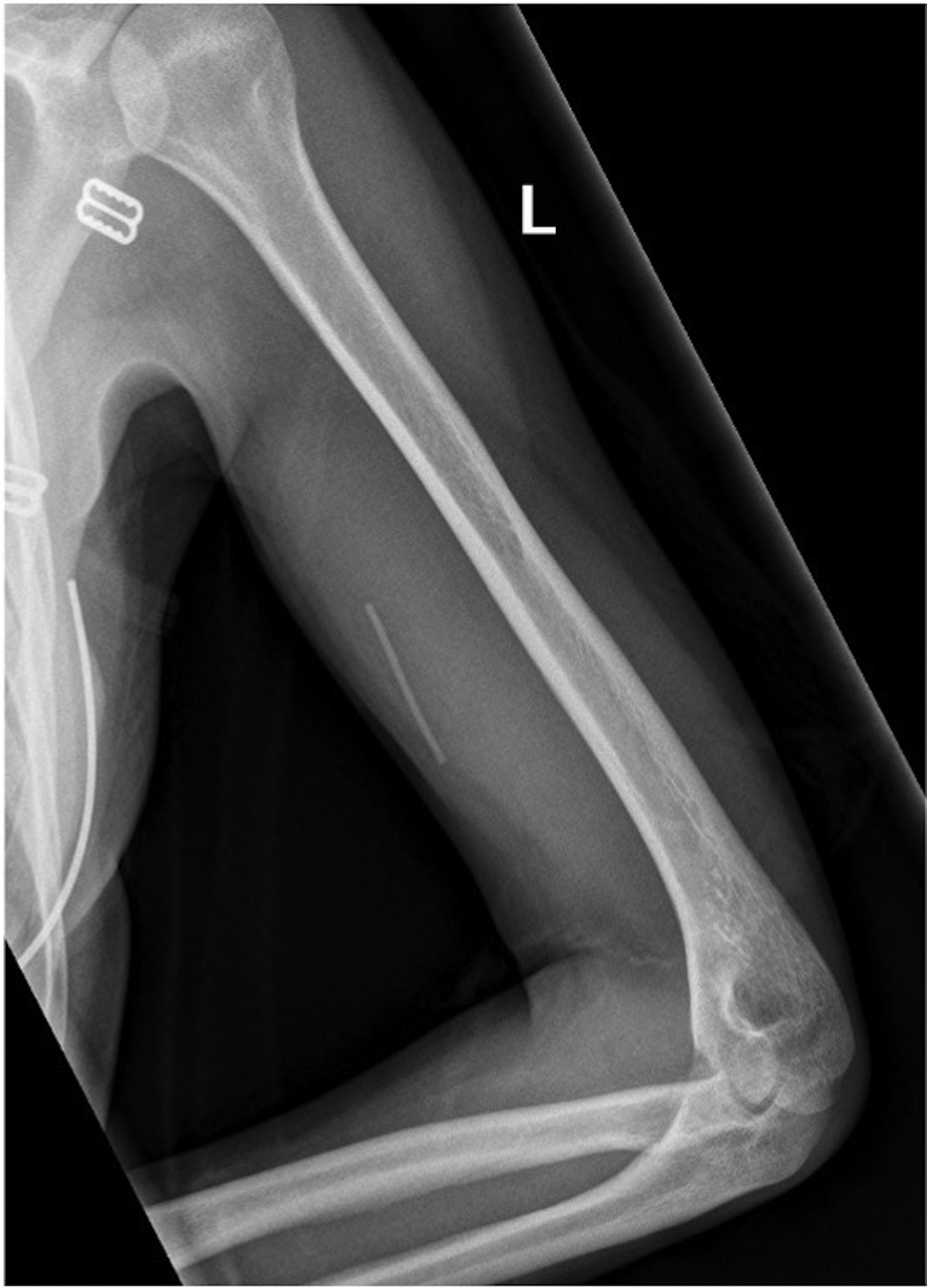
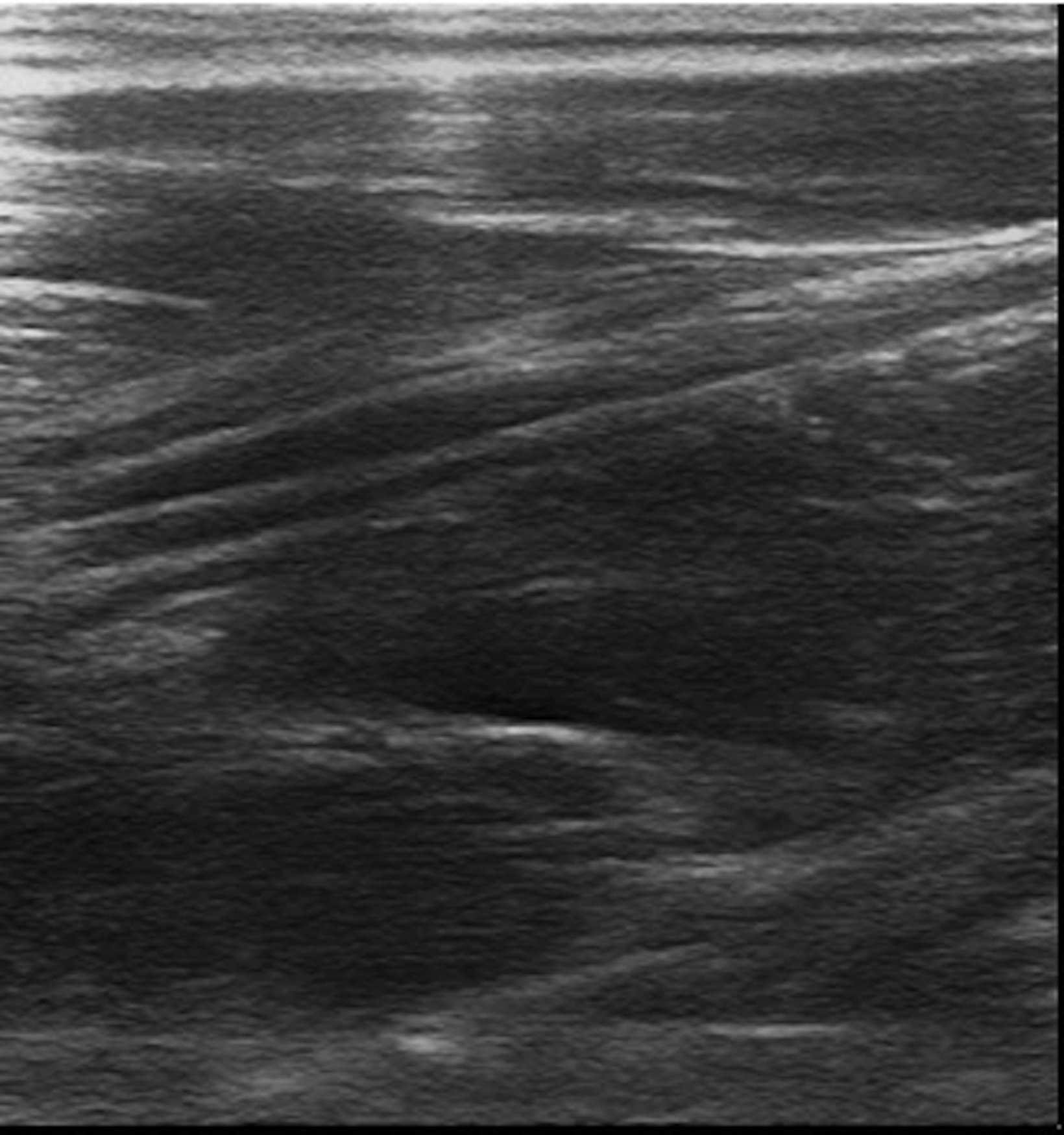
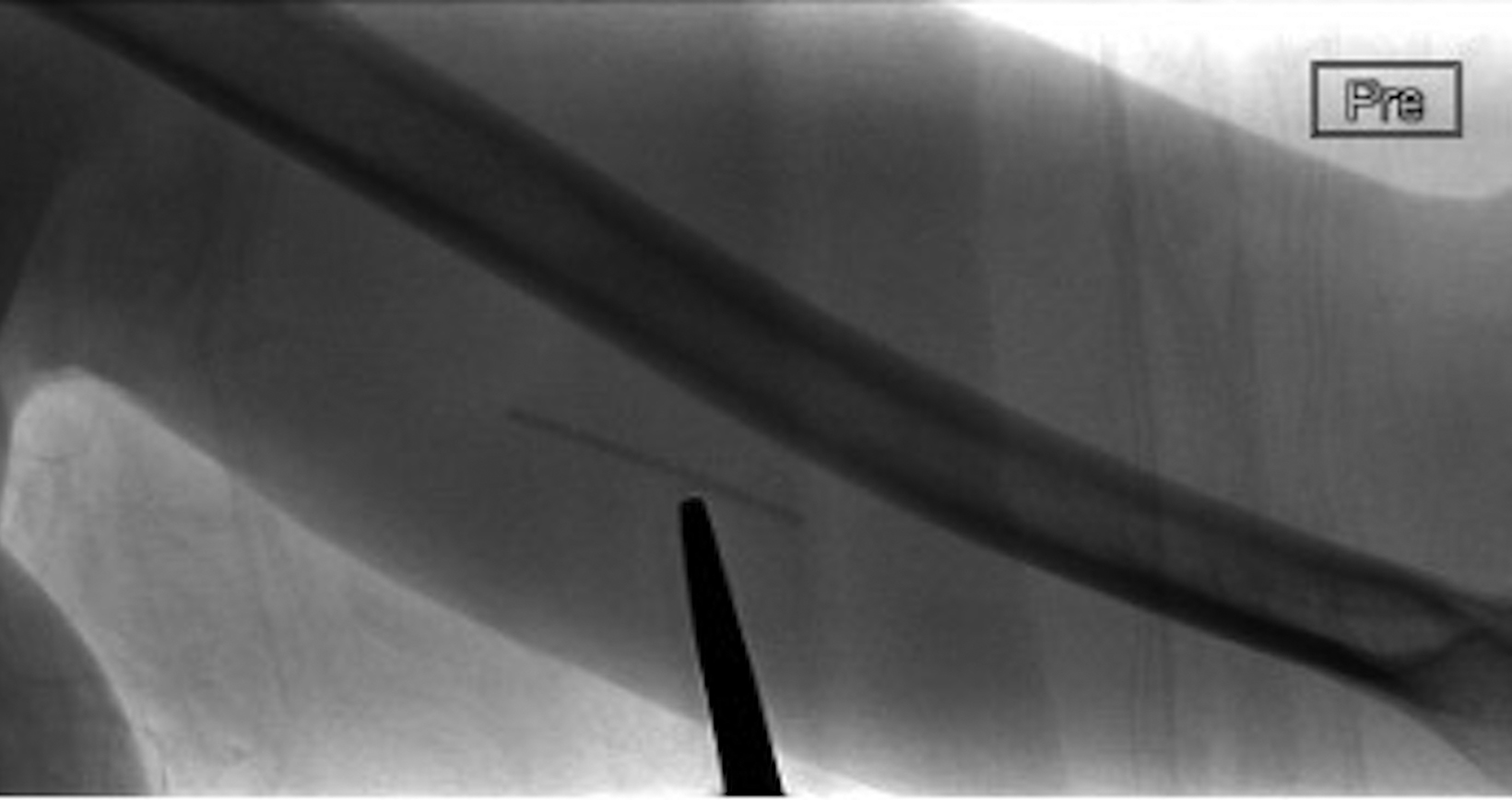
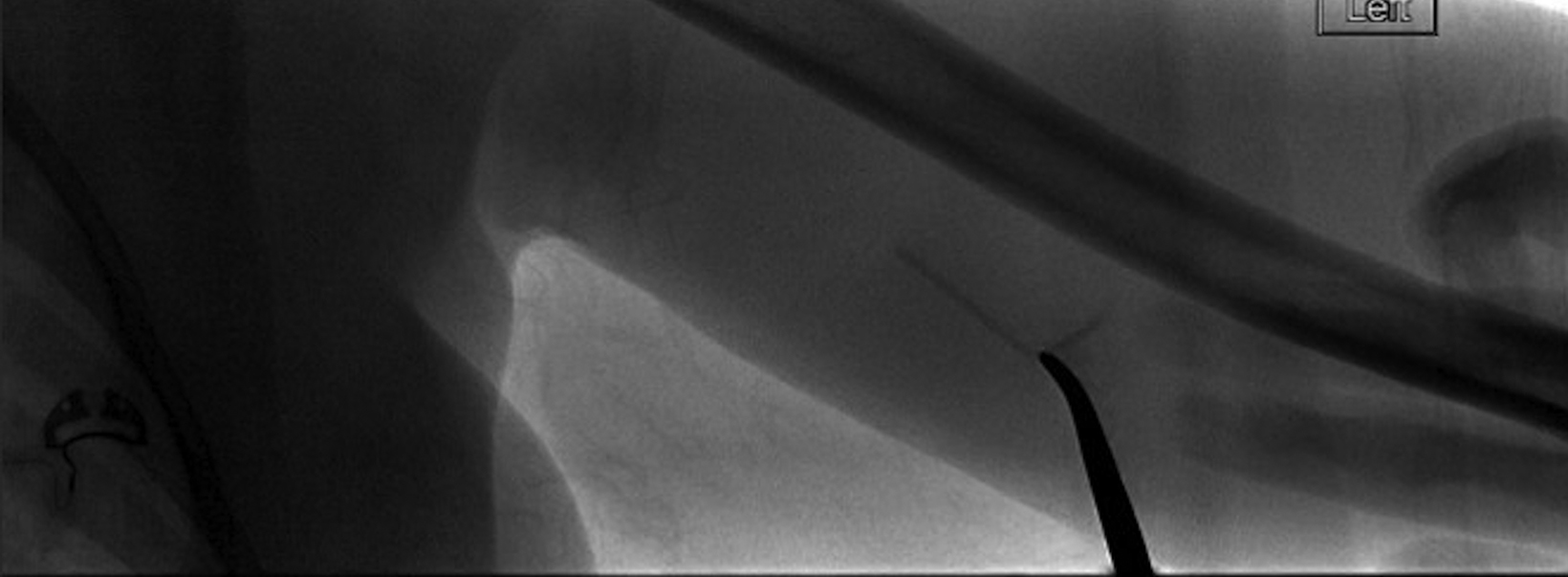
Radiographs of the left humerus demonstrated 4 cm linear Nexplanon implant in the soft tissues of the upper arm (Figure 1). The patient was referred to interventional radiology for image-guided removal. The position of the contraceptive implant in the superficial soft tissues of the medial upper arm was confirmed using ultrasound (Figure 2). Preprocedure localization was performed (Figure 3) and the device was removed under image-guidance using blunt dissection (Figure 4). Complete removal was confirmed under fluoroscopy.
Diagnosis
Deeply implanted subdermal contraceptive device
Discussion
According to a 2021 Guttmacher Institute report, approximately 1.5 million women in the United States use a contraceptive implant. Nexplanon (Merck, Kenilworth, New Jersey), a 4 cm linear and radio-opaque etonogestrel contraceptive, is highly effective, safe, and nonteratogenic with a Pearl Index of 0. The Pearl Index measures the number of pregnancies per 100 women per year using the contraceptive method.1 The device is placed sub-dermally in the medial aspect of the nondominant arm 8-10 cm above the medial epicondyle of the humerus and 3-5 cm posterior to the sulcus. Located between the biceps and triceps, the sulcus contains the median nerve, ulnar nerve, basilic vein, and brachial artery. Nexplanon is long-acting, reversible, and effective for up to three years.1
Appropriately positioned subdermal implants are typically easily palpable. Nonpalpable implants are usually placed deep, have migrated (most commonly to the bicep or axillary region), or because of weight gain. Such implants should be localized using ultrasound or radiography.
The Nexplanon Observational Risk Assessment study reported the incidence of incorrect placements to be 12.6 per 1000 insertions.2 Incorrectly placed implants can lead to vascular injury, median and ulnar neuropathy, and migration to the pulmonary artery (3.17 per 100,000 implants). Migration to pulmonary vasculature, a very rare occurrence, can be life threatening. Patients may present without any symptoms or with chest pain and/or dyspnea.1
Abnormal menstrual bleeding is the most common reason for early removal of Nexplanon, although insertion-site pain, cellulitis, neurovascular injury, and hematoma are some other reported complications. The device is contraindicated in patients with breast cancer, severe liver disease, and deep vein thrombosis.1
Appropriately placed subdermal implants are usually removed under local anesthesia without image-guidance. Nonpalpable implants may be removed surgically or under imaging guidance. Interventional radiologists are increasingly being integrated in the multidisciplinary management of malpositioned implants.
Patients undergo preprocedure radiography to ensure the device is in the upper arm. The implant is localized under fluoroscopic guidance, and a small incision (∼1cm) is made over the peripheral aspect of the implant. The implant is retrieved using blunt dissection. The procedure is usually performed with mild/moderate sedation but can also be performed with local anesthesia only.
Injecting lidocaine around an implant located next to vital structures permits easy removal, decreases the risk of damage to surounding structures, and provides additional anesthesia.3 Both measuring the length of the removed implant and postprocedure fluoroscopy are essential to confirm complete removal. Although surgical removal has been described in the literature,4 fluoroscopy-guided removal allows for precise localization, confirmation of complete removal, and decreased risk of injury to surrounding structures.
If the device is not evident at radiography or ultrasound, serum etonogestrel levels can be obtained before proceeding with other imaging modalities, such as CT or MRI, to ensure that the implant is present. In the rare cases of migration to the pulmonary artery, endovascular retrieval can be attempted. Early detection is key, as endothelialization of the device makes retrieval more difficult and may warrant surgical intervention.3 Nonradiopaque implants (Implanon, Merck; Norplant, Wyeth Pharmaceuticals) may be localized using US, CT, MRI, and compression film mammography.
Conclusion
Imaging of nonpalpable contraceptive implants is essential for appropriate localization and development of a management plan. Interventional radiologists should be a part of multi-disciplinary management of deep implant removals. Fluoroscopy-guided removal should be considered as a therapeutic option in patients with deep or migrated implants.
References
Citation
NK K, TM C. Removal of Subdermal Contraceptive Device. Appl Radiol. 2023;(4):44-46.
June 23, 2023