Radiation-associated Angiosarcoma of the Breast: A Clinicopathological and Multimodality Imaging Review
Images

Abstract
Patients diagnosed with breast cancer who have been treated with breast conserving surgery and radiation therapy have an increased risk of developing radiation-associated angiosarcoma of the breast. With the increased use of breast conserving surgery and radiation therapy in the management of breast cancer, greater awareness and understanding of the disease is required. This review describes the epidemiology, etiology, clinical presentation, imaging features, differential diagnosis, and histopathological features of radiation-associated angiosarcoma of the breast. Multimodality imaging includes mammography, ultrasound, and magnetic resonance imaging. Moreover, we highlight key clinical practice points for radiologists regarding identification and management of the disease.
Key words: breast imaging, breast cancer, angiosarcoma, radiation-associated angiosarcoma
Introduction
Angiosarcomas are malignant tumors that originate from endothelial cells lining vascular channels. They are a rare histologic subtype of soft-tissue sarcomas and they represent only 1 to 2% of all soft-tissue sarcomas.1 The breast is a common tissue in which angiosarcoma may arise. Angiosarcoma of the breast may arise spontaneously (primary angiosarcoma of the breast) or it may arise secondary to a biological insult (secondary angiosarcoma of the breast), such as radiation therapy (radiation-associated angiosarcoma of the breast [RAAS], Box 1).2-4
In recent years, RAAS has become increasingly reported and it has been suggested to be associated with the increased use of breast conserving surgery and radiation therapy in the management of breast cancer.2,5 Diagnosis of the disease yields poor prognosis with significant metastatic potential.5 As such, radiologists’ ability to appropriately recognize RAAS is integral. We provide a comprehensive review of RAAS as well as a multimodality imaging review of the disease.
Epidemiology
Risk and incidence
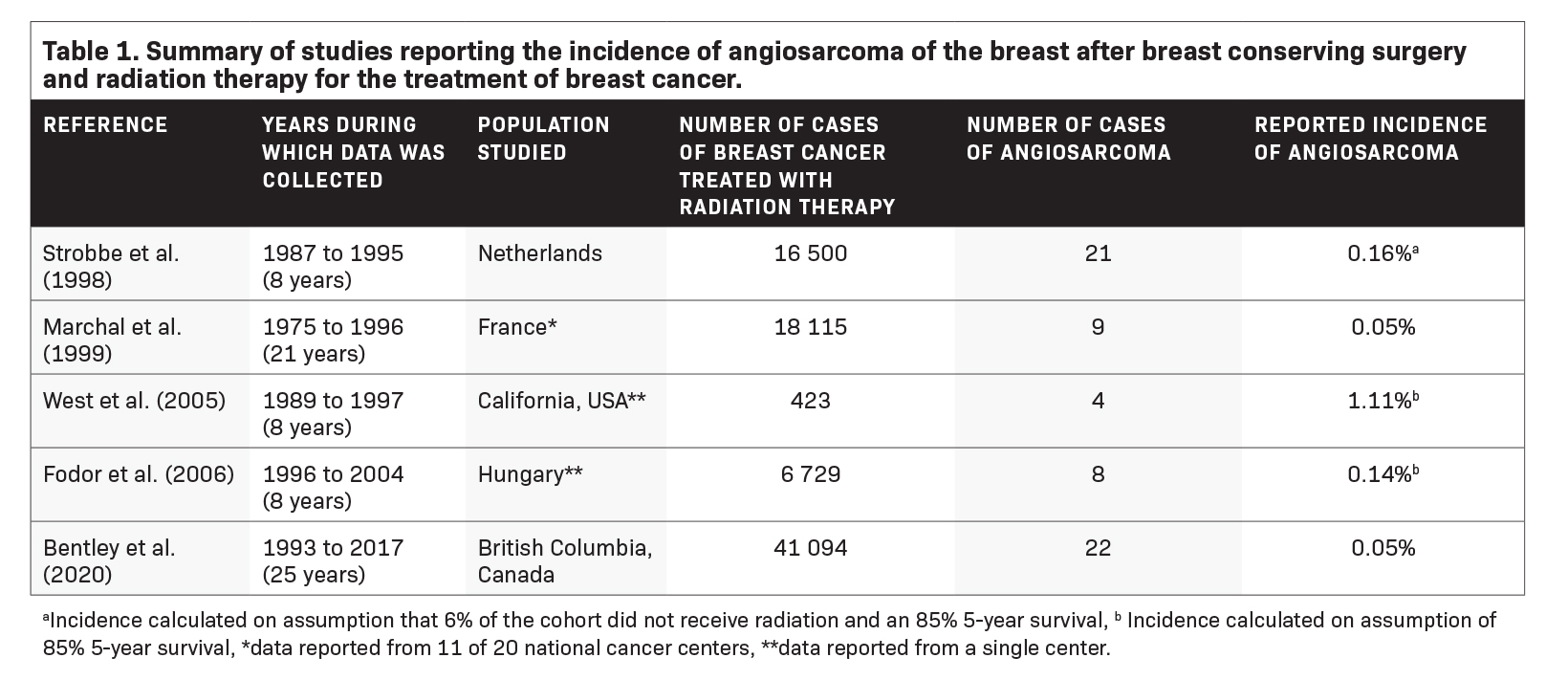
Studies have demonstrated that patients diagnosed with breast cancer who are treated with breast conserving surgery and radiation therapy have an increased risk of developing RAAS as compared to those patients who did not receive radiation therapy.6 Large population-based studies utilizing the Surveillance, Epidemiology, and End Results (SEER) database have reported the incidence rate of RAAS to be 7.6 per 100 000 person years.7 Five studies have estimated the incidence proportion of RAAS, with the incidence proportion reported varying from 0.05% to 1.11% (Table 1).8-12 While certain studies have demonstrated an increased number of cases of RAAS over time, the incidence proportion of RAAS reported over time has not increased.12,13
Etiology
The etiology of RAAS remains poorly understood. Genome instability arising from DNA damage and direct tumor induction arising from radiation therapy through mutations of relevant cancer-related genes are thought to be central to pathogenesis.14 Prolonged cellular stimulation during tissue repair associated with radiation-induced ischemic changes may also have a role in the development of disease. Malignant transformation of pre-existing primary benign lesions after radiation therapy has also been proposed.14,15
The risk of RAAS in BRCA 1/2 patients has also been evaluated. Deficiencies in the DNA repair mechanisms of these patients have been hypothesized to increase radiosensitivity and susceptibility to carcinogenesis in surviving cells.14 Kadouri et al (2013) observed a high frequency of BRCA 1/2 mutations among patients diagnosed with RAAS.16 Nonetheless, while a two-fold increased risk of RAAS among BRCA 1/2 carriers was estimated, given the rarity of the disease this increased risk was not found to be significant as compared to patients without the mutation.16 BRCA 1/2 mutations should thus not be considered in the decision to pursue treatment with radiation therapy, and they ought not to preclude its use.16
The use of chemotherapy has not been identified as a risk factor for the diagnosis of RAAS. A systematic review of radiation-associated sarcomas completed by Sheth et al (2012) did not find any association between the use of chemotherapy and the development of the disease.14
Clinical Presentation
Radiation Latency
Huang and Mackillop (2001) demonstrated that the risk of developing RAAS is increased within 10 years after radiation therapy, with peak risk being between 5 and 10 years after radiation therapy.6 Most studies have reported a median radiation latency, defined as the interval between radiation therapy and the development of RAAS, of approximately 7 years.9,13,12,17-19 Nonetheless, radiation latency may vary greatly, with a range of 11 months to 24 years being reported.20
Age at Presentation
RAAS generally presents in older women. The median age at presentation is 70 years, with a range of 36 to 92 years being reported.20
Clinical Features
The clinical presentation of RAAS varies. RAAS most often affects the dermis, though the disease may also occasionally affect the breast parenchyma.2,3 Skin changes are often nonspecific and may include discoloration, bruising, nodularity, induration, thickening, fibrosis, telangiectasia, and/or bleeding of the skin. Discoloration associated with RAAS is often described as faint purple, blue, or black or a bruise-like change in the skin. 3,21 A palpable lesion underlying skin changes may also be present.13 While skin changes are most often localized to the vicinity of the previous surgical site or site of radiation therapy, there may also be diffuse involvement of the breast or adjacent area.3 Thorough clinical assessment is imperative to avoid the omission of satellite lesions, which are oftentimes identified.21 Given the nonspecific nature of the clinical presentation of the disease, diagnosis is often challenging, as skin changes or lesions may be easily mistaken for radiodermatitis, trauma, or another cutaneous lesion.4,21 Of the 21 patients observed by Strobbe et al (1998), 6 patients were reported to have delays in appropriate diagnosis, which was attributed to unfamiliarity with the disease.8 Documentation of skin changes identified by clinicians or by technologists during image acquisition is thus integral to ensure appropriate diagnosis.
Imaging Features
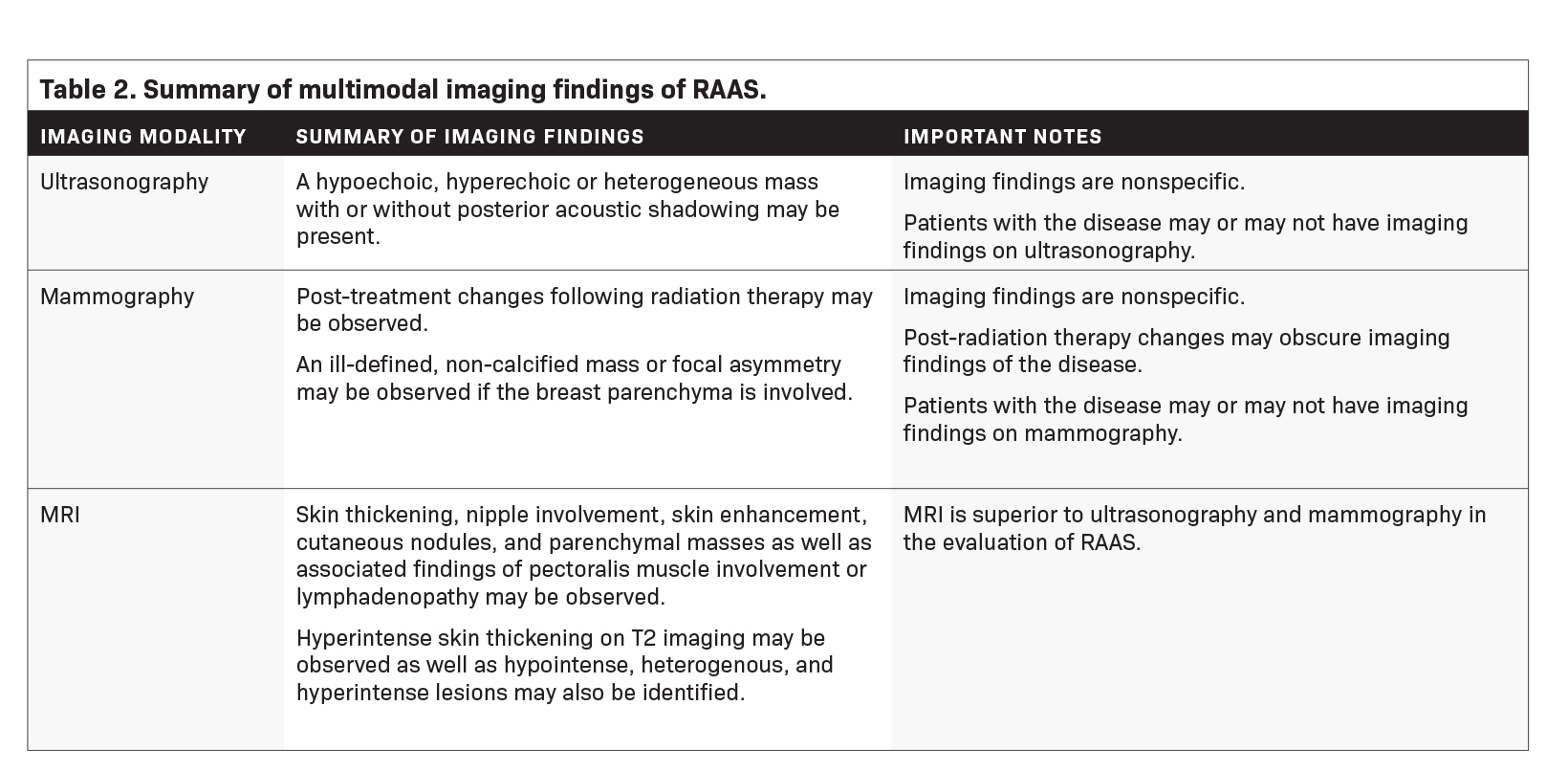
Imaging features of RAAS are summarized in Table 2.
Mammography
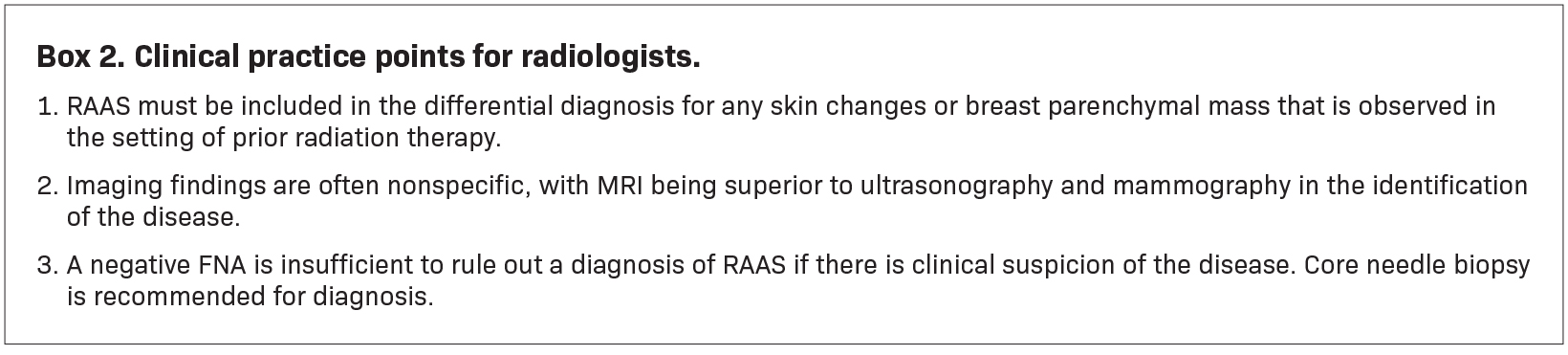
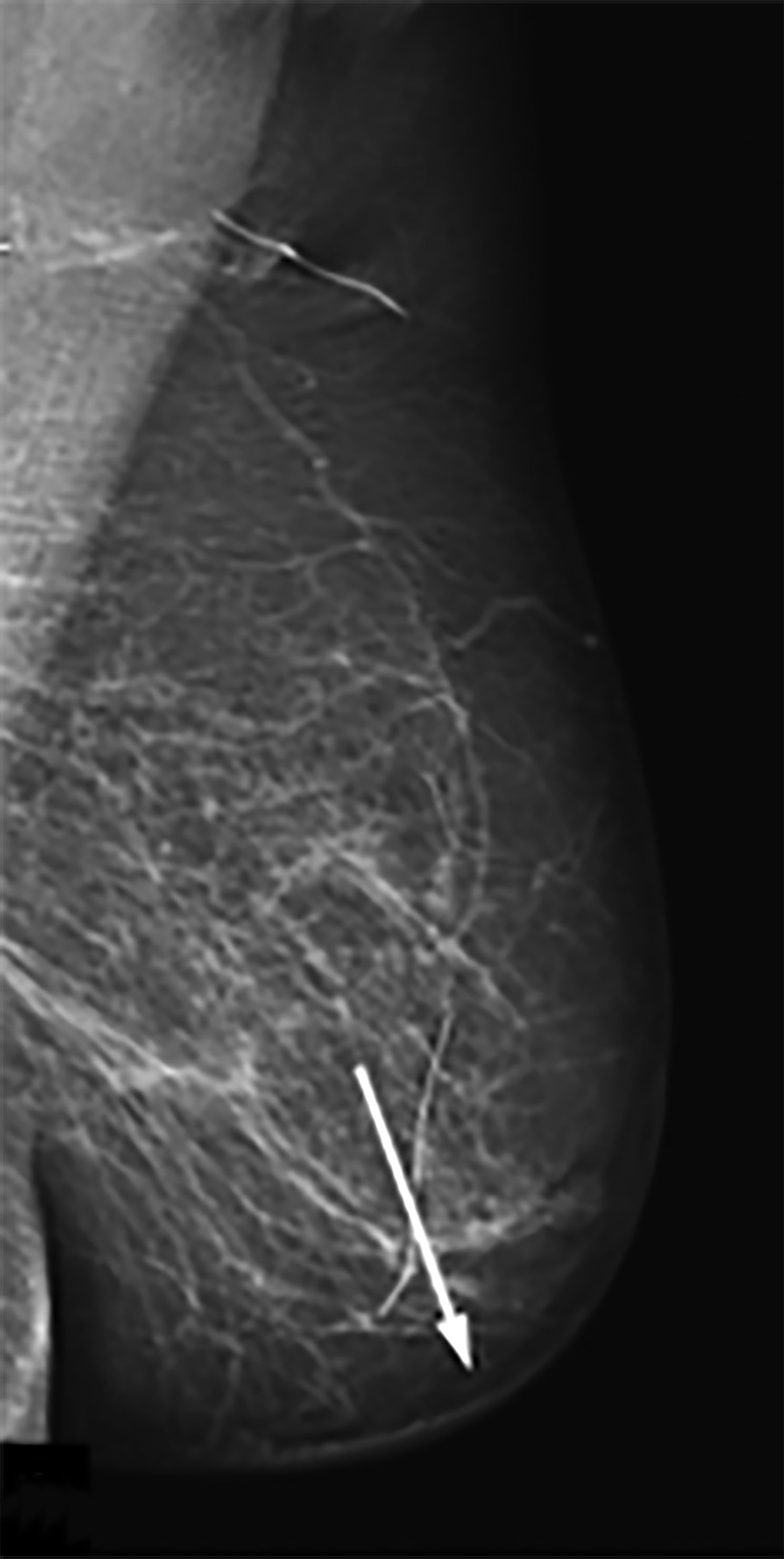
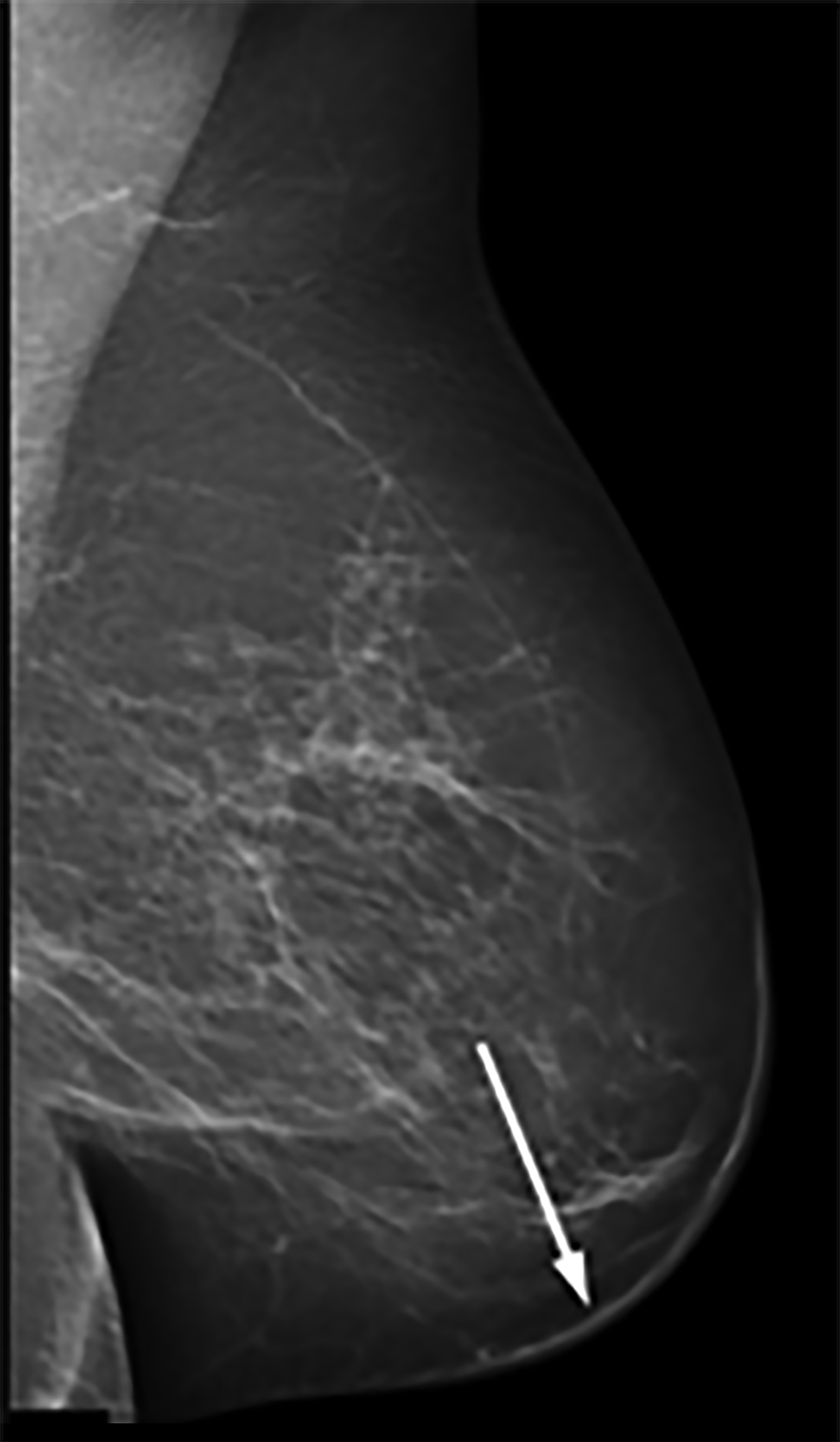
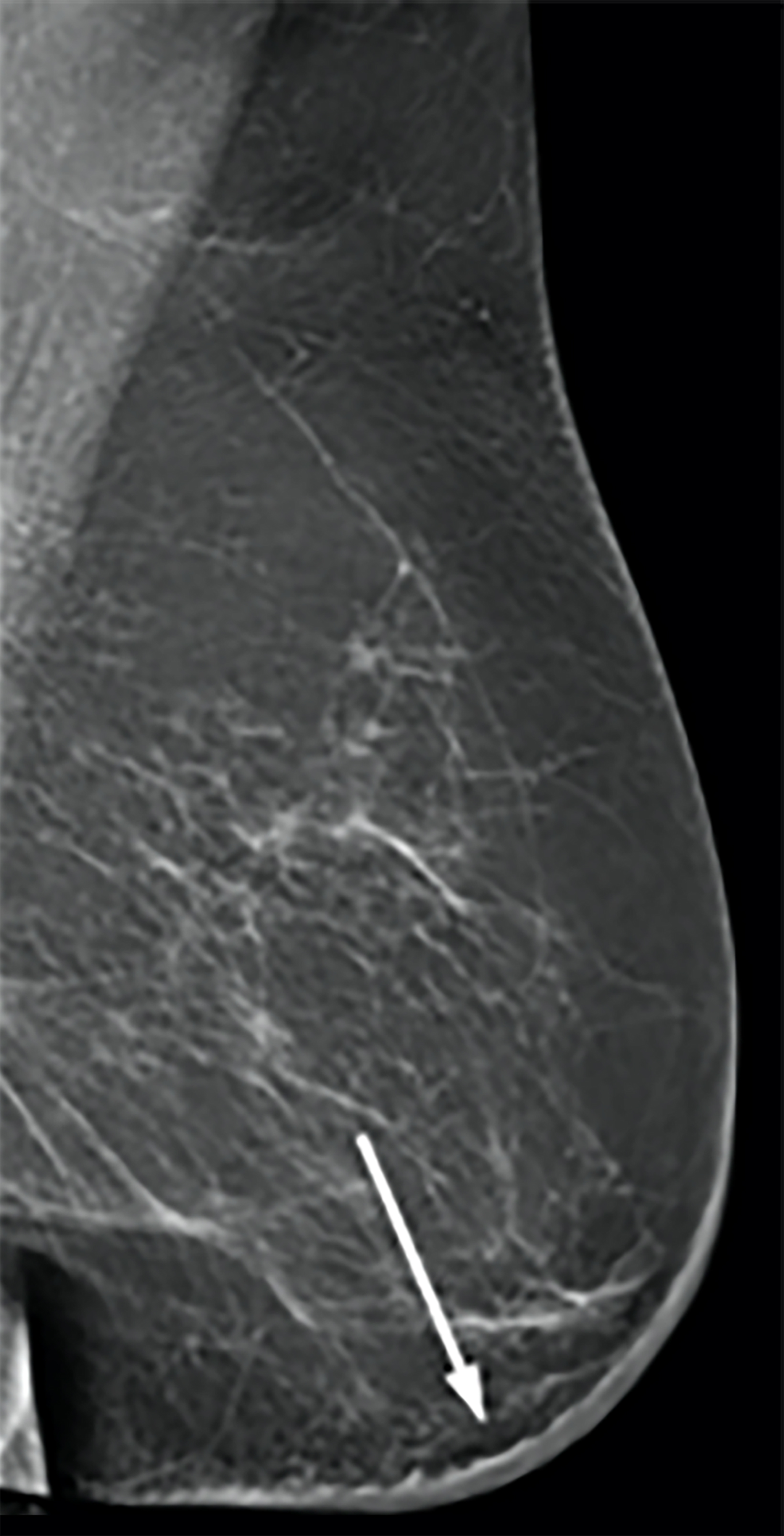
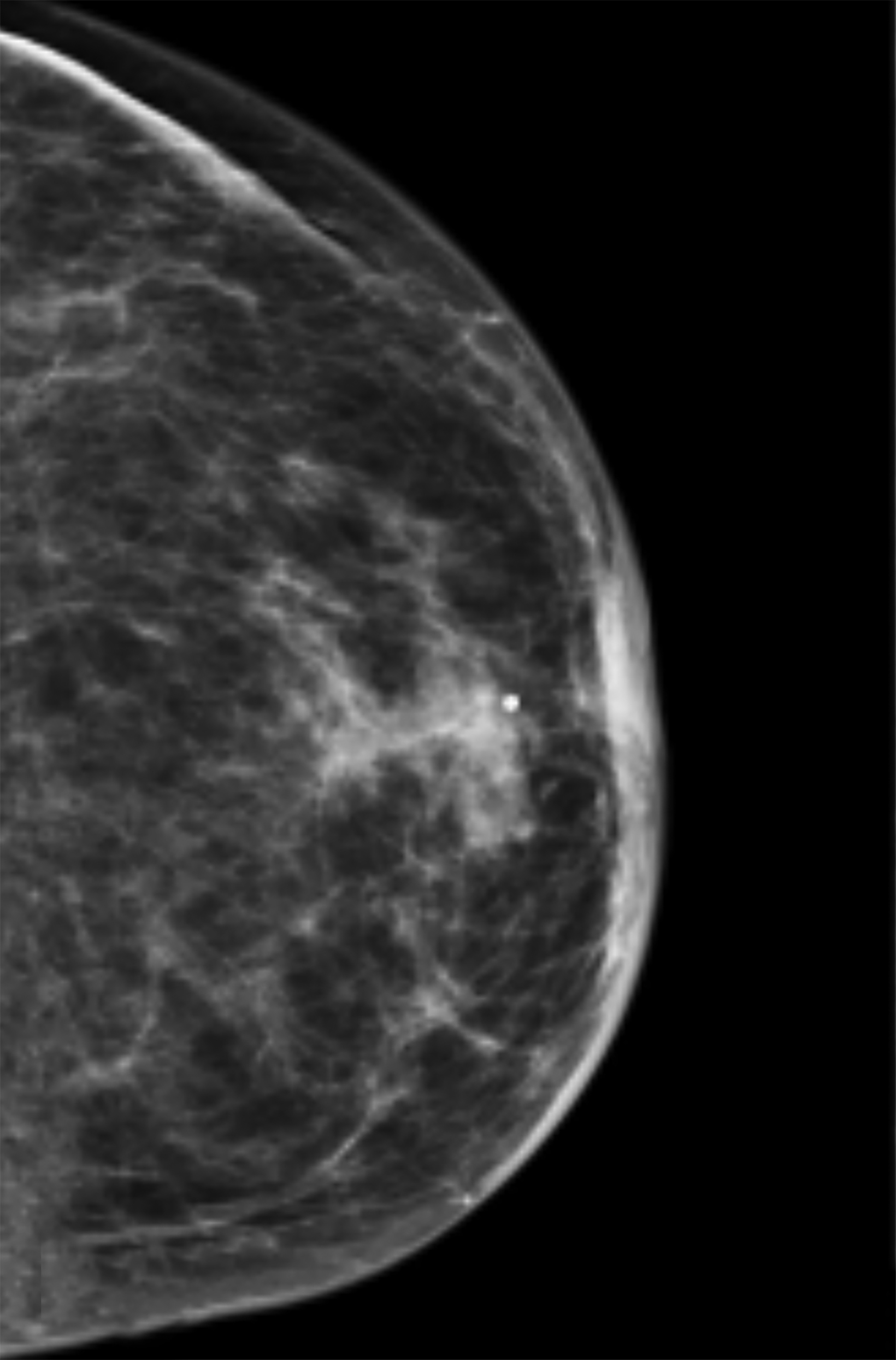
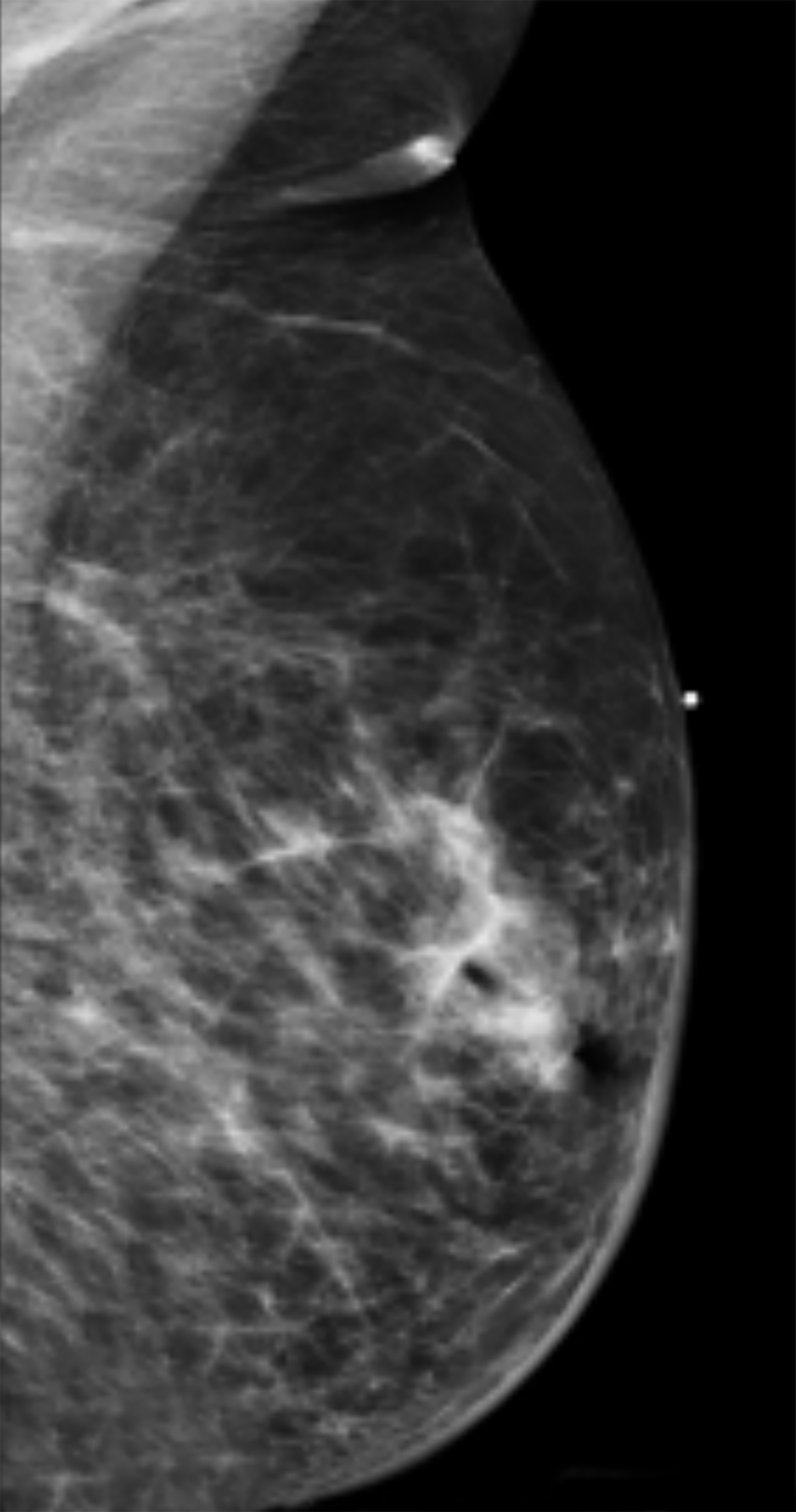
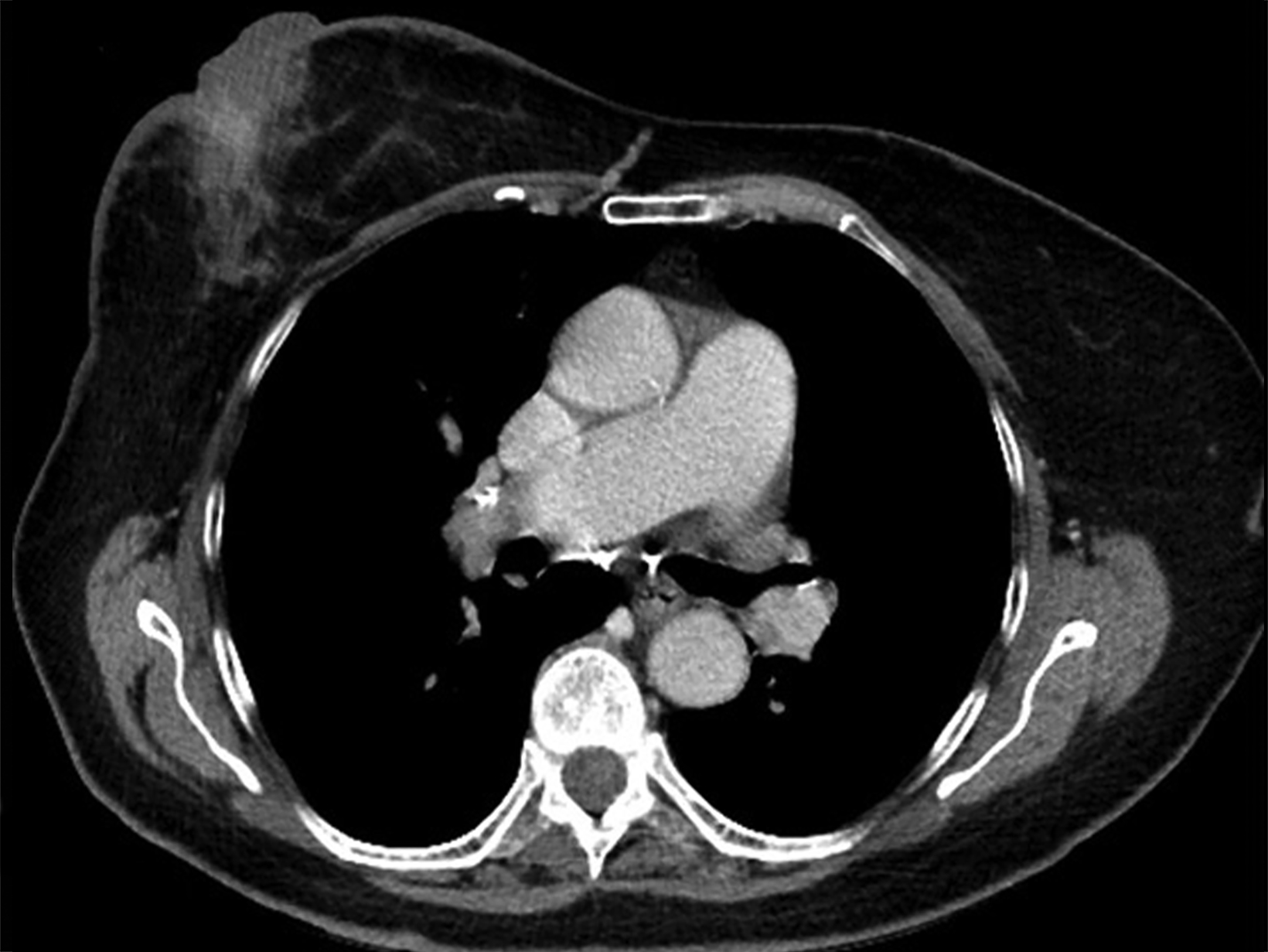
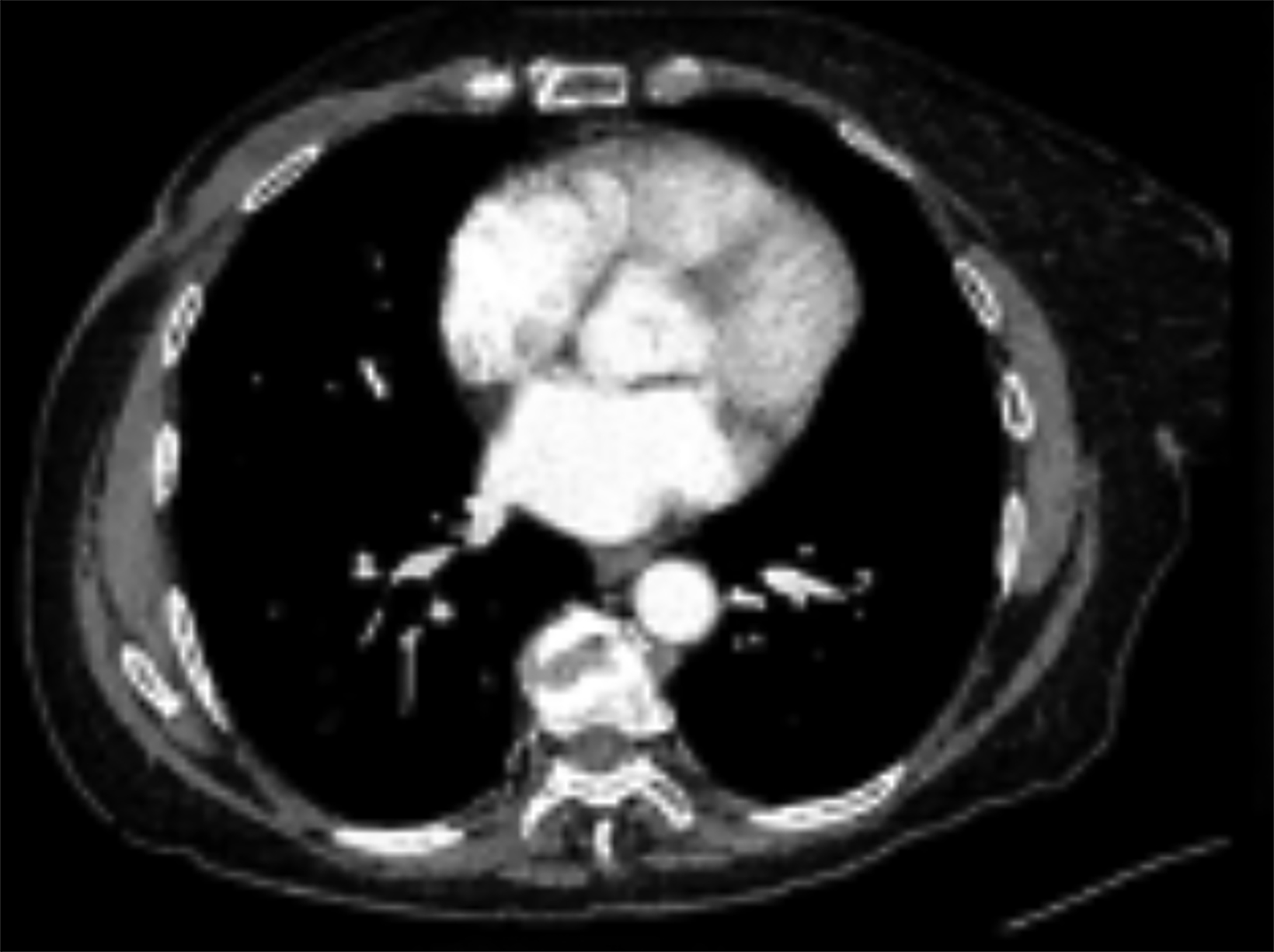
Mammography findings of RAAS are nonspecific. Often only post-treatment changes after radiation therapy may be seen mammographically, including skin thickening, skin retraction, and architectural distortion (Figure 1).2,22-24 These post-treatment changes may obscure other mammographic findings of the disease.24 If the breast parenchyma is involved, an ill-defined, non-calcified mass or focal asymmetry of the breast may be observed (Figure 2).25 Mammographic imaging has also been reported to be negative despite skin changes being observed clinically.5,26-28
Ultrasonography
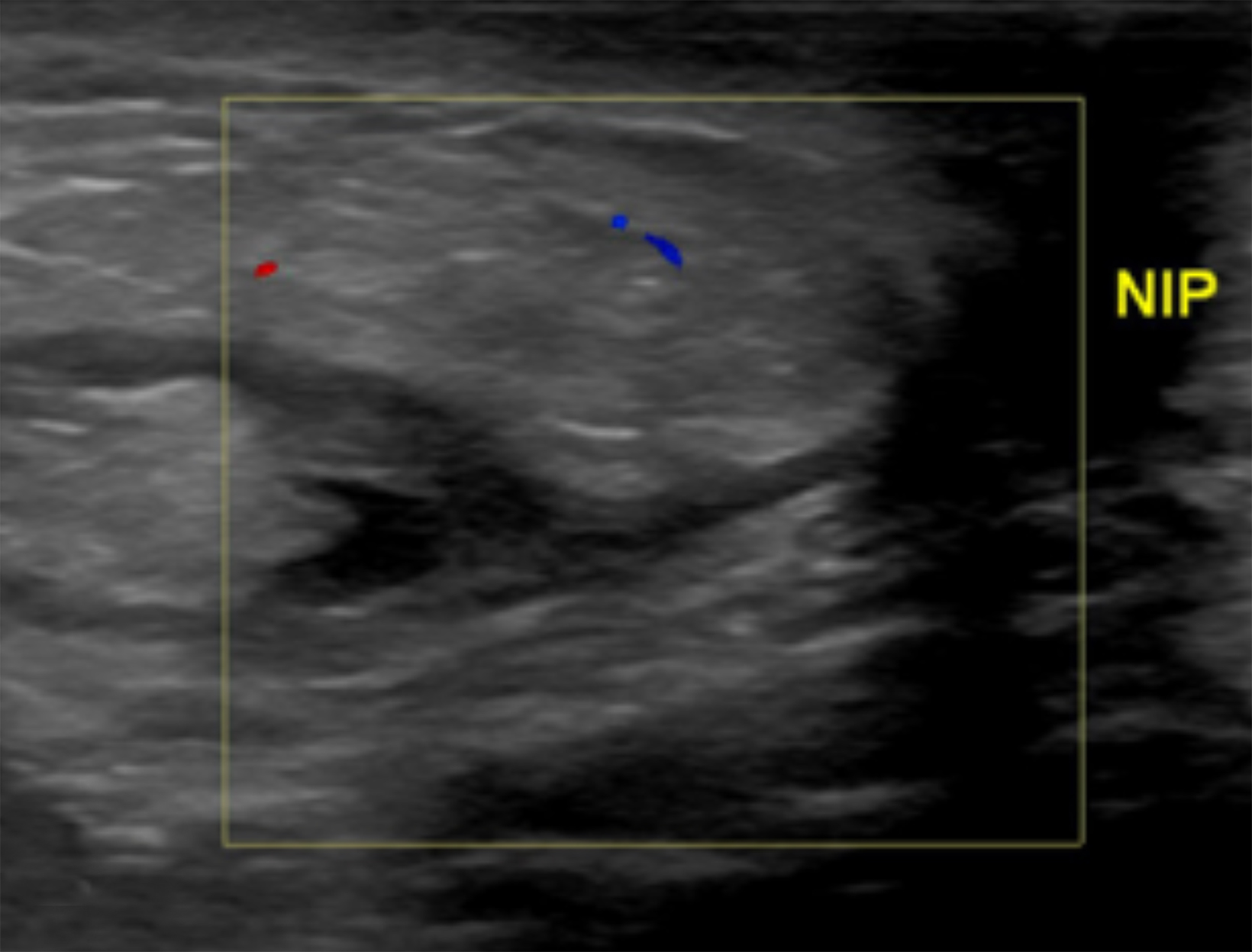
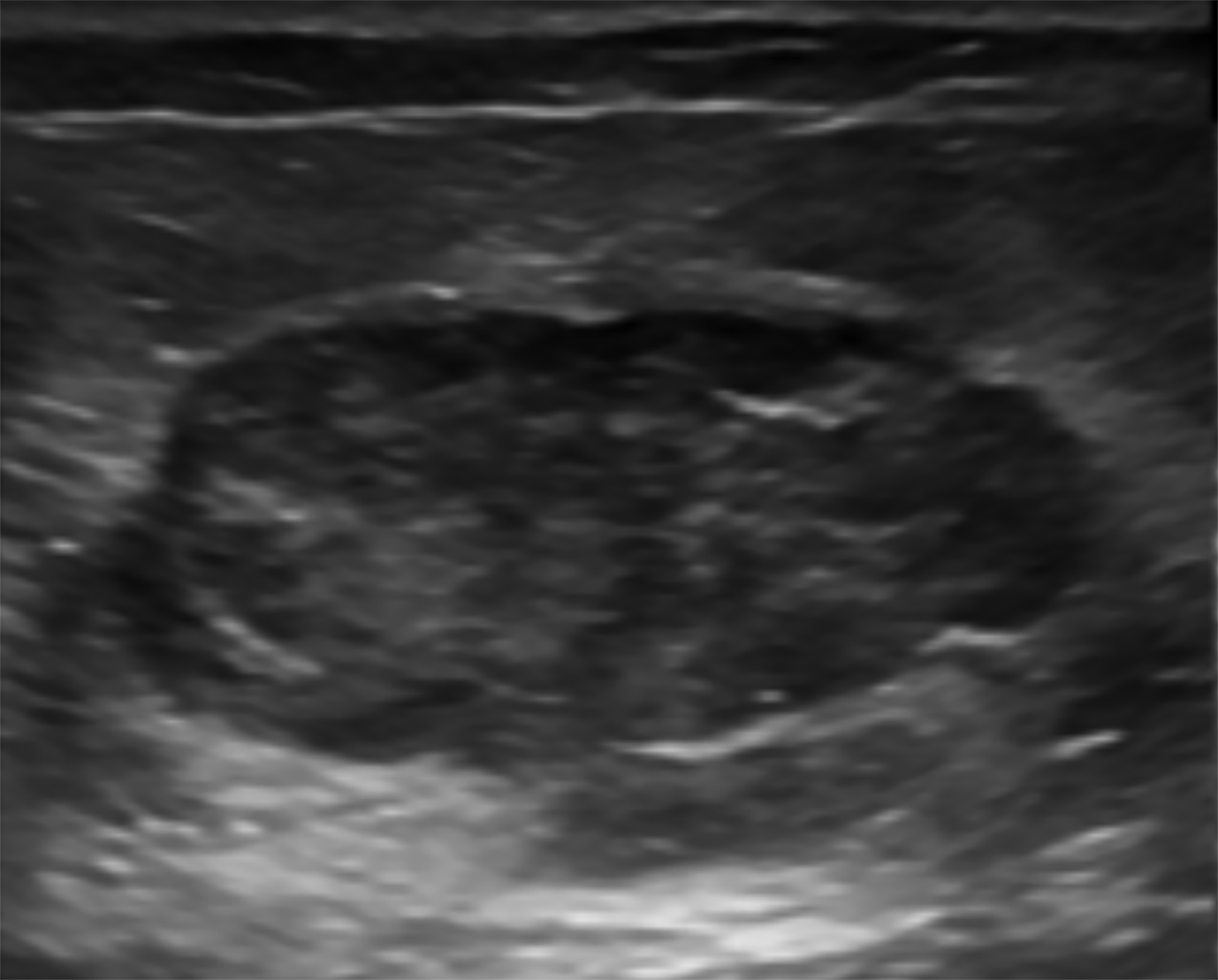
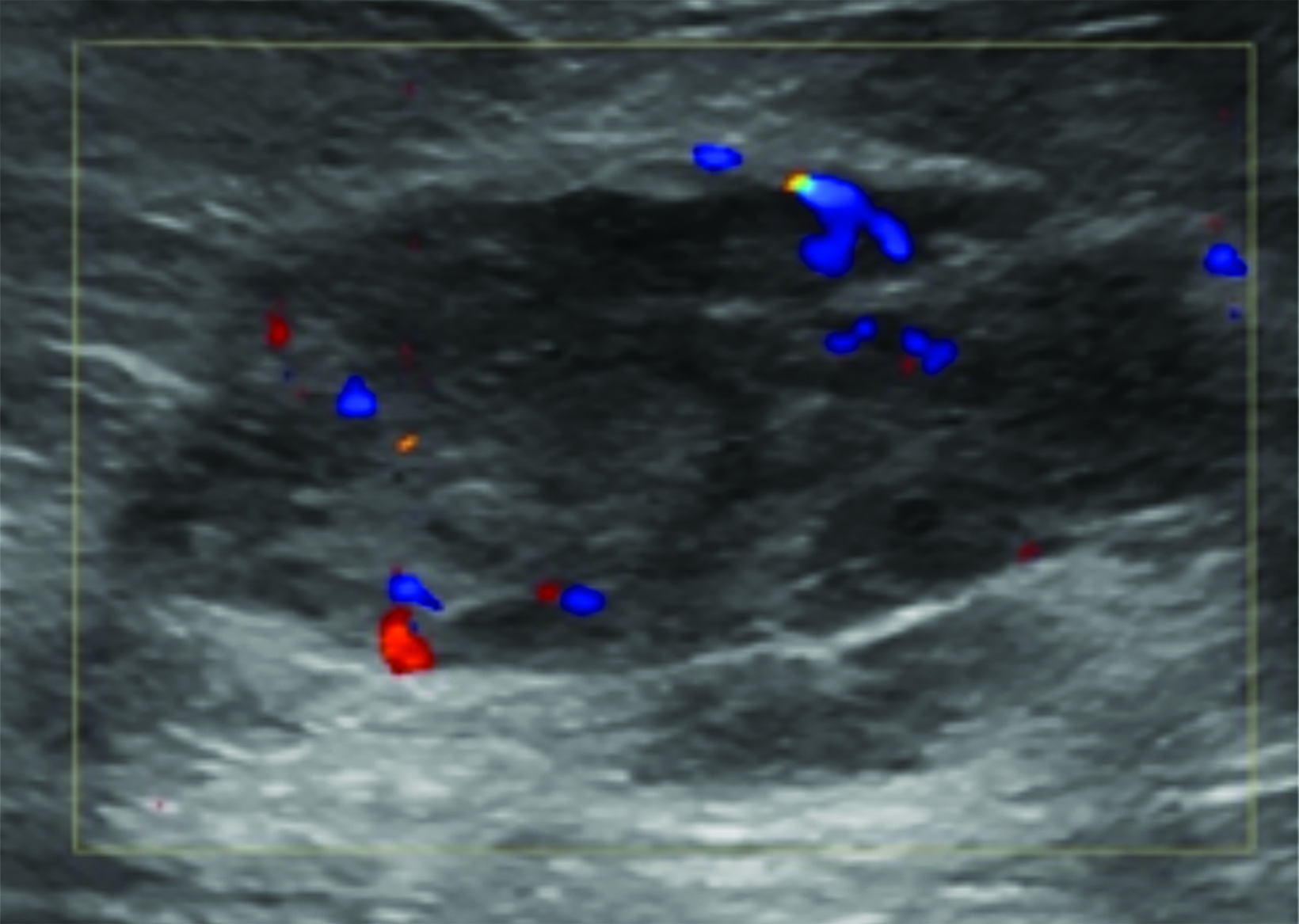
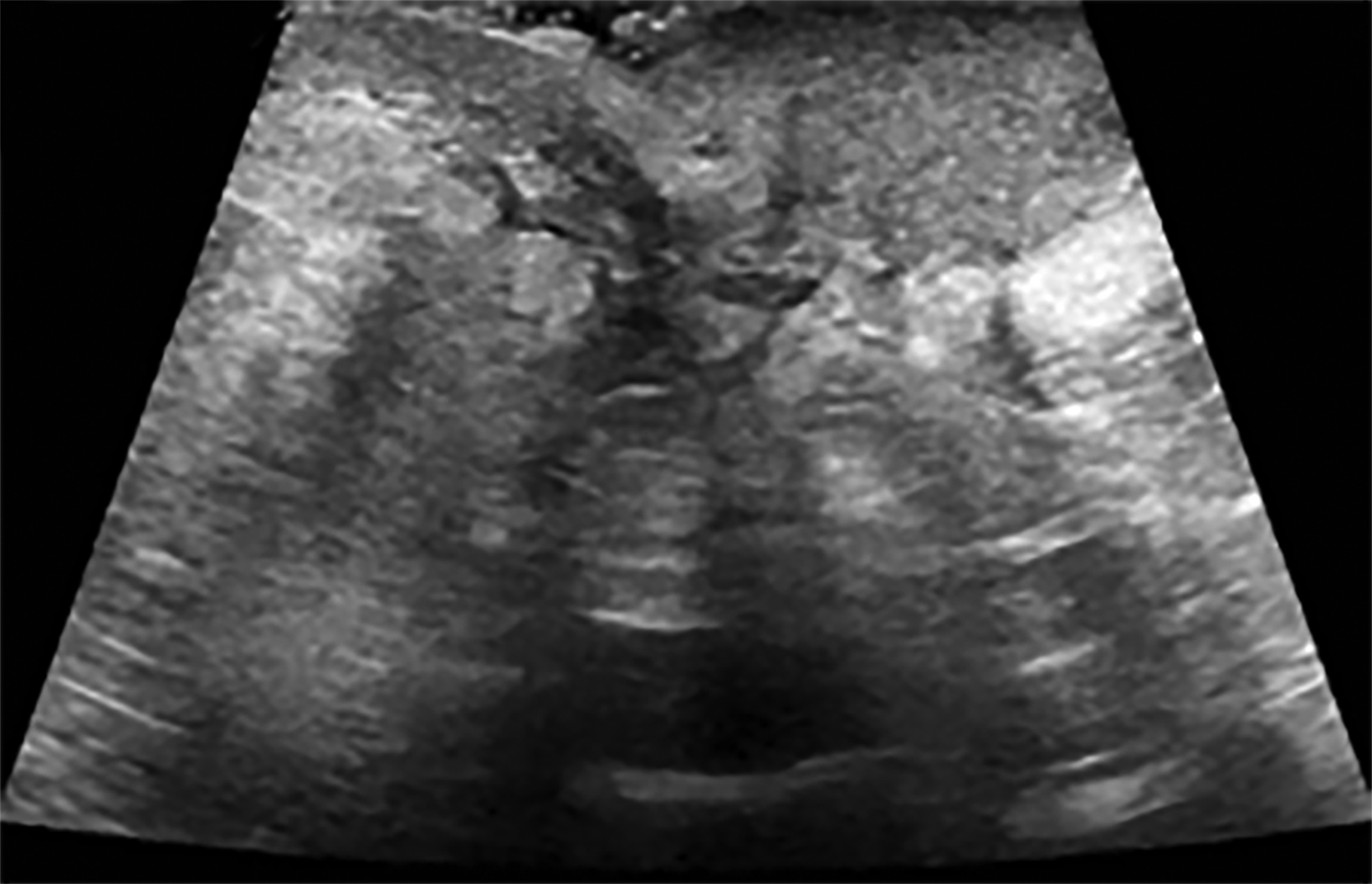
Ultrasound may serve as a useful initial screening modality at sites of previous radiation therapy or previous tumor localization. Ultrasound may also provide temporal comparison if patients develop clinically significant findings at the site of previous radiation therapy, such as skin nodularity or thickening.29
Ultrasound imaging findings of RAAS are generally nonspecific. Ultrasound may demonstrate a hypoechoic, hyperechoic, or heterogeneous mass with or without acoustic shadowing. Color Doppler flowmetry may be useful if enhanced vascularity is observed (Figure 3).2,25 Of the 6 patients observed by Chikarmane et al (2015), 3 patients underwent ultrasound imaging, which revealed skin thickening and non-specific post-radiation therapy skin changes and underlying irregular masses.22
Magnetic Resonance Imaging
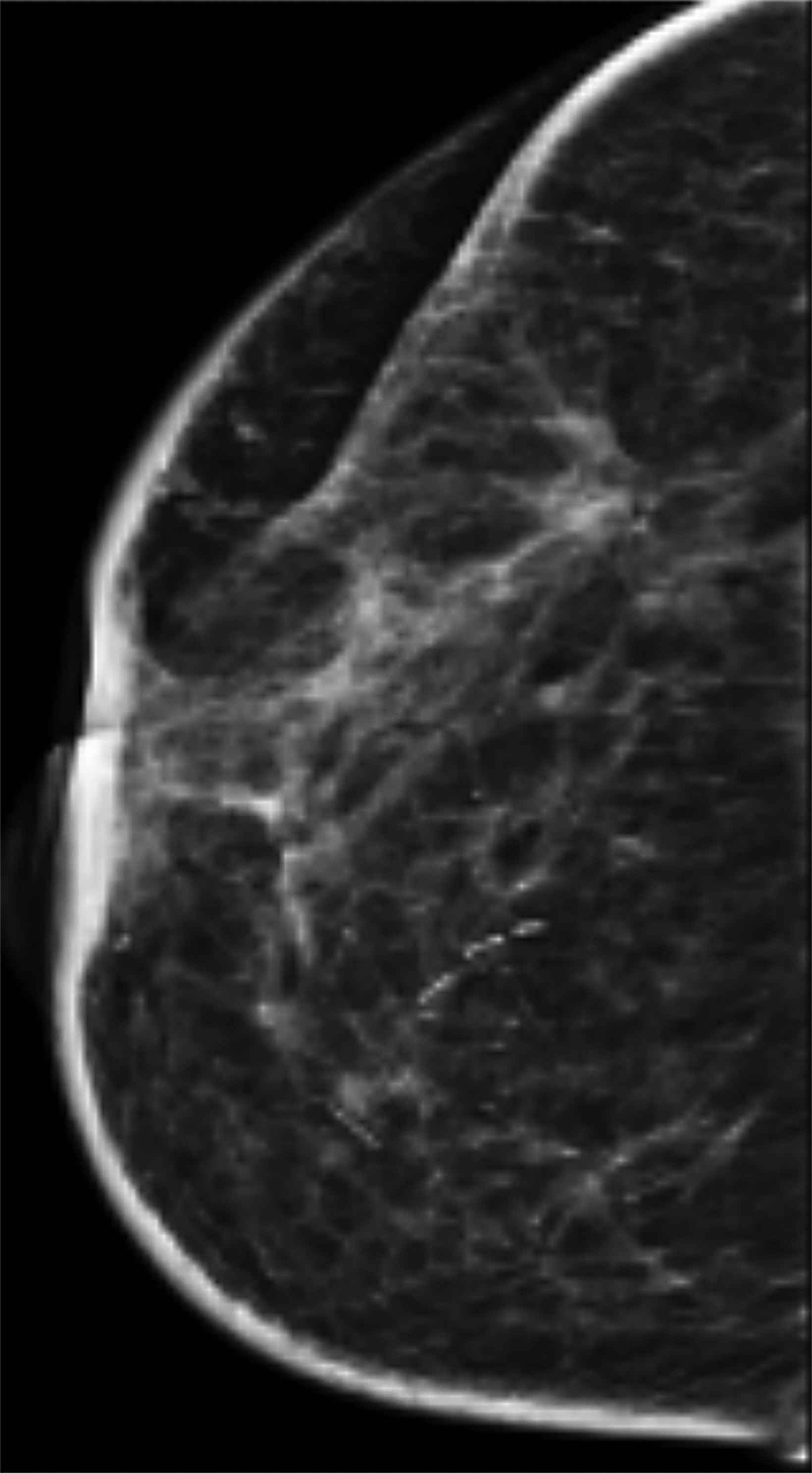
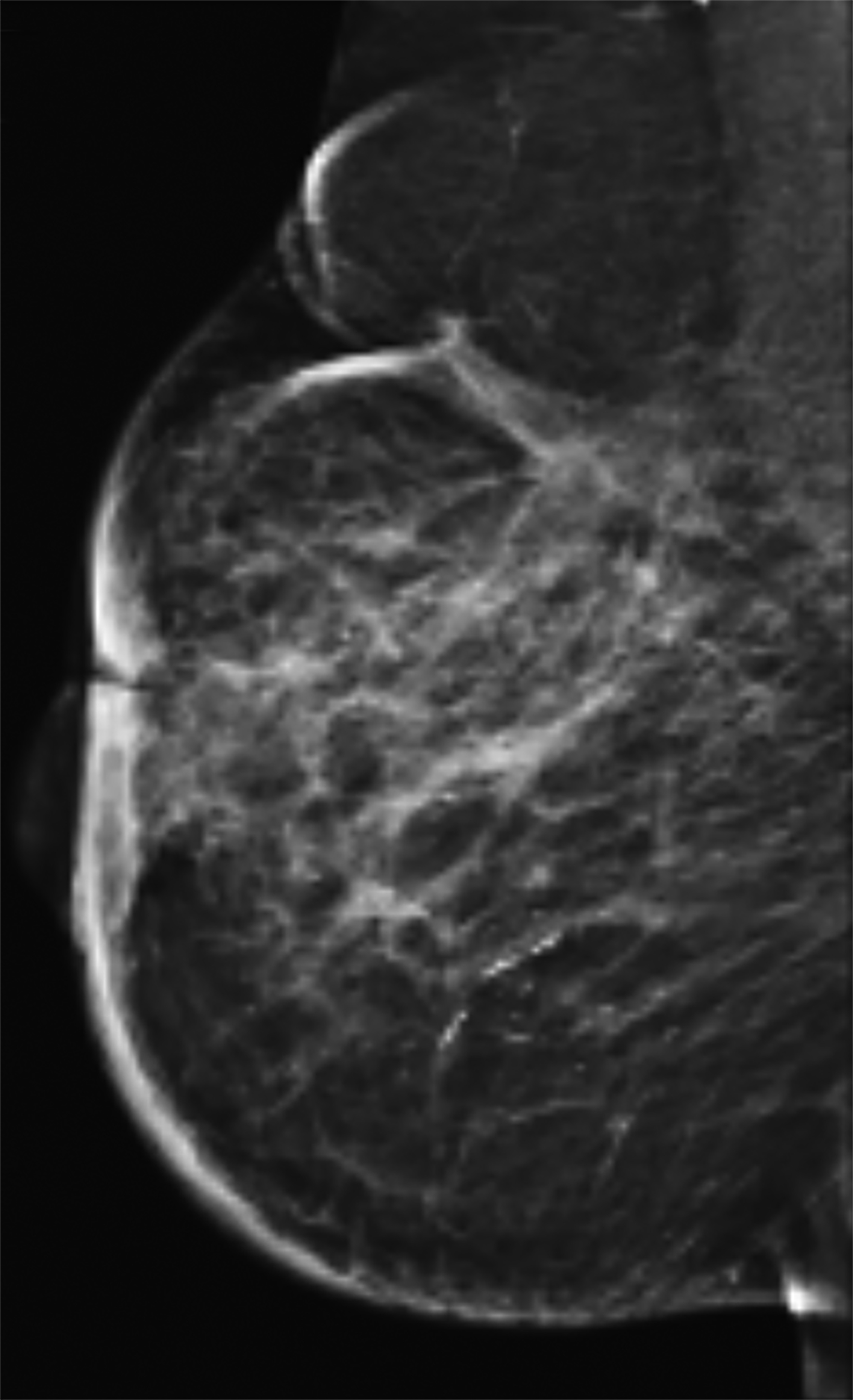
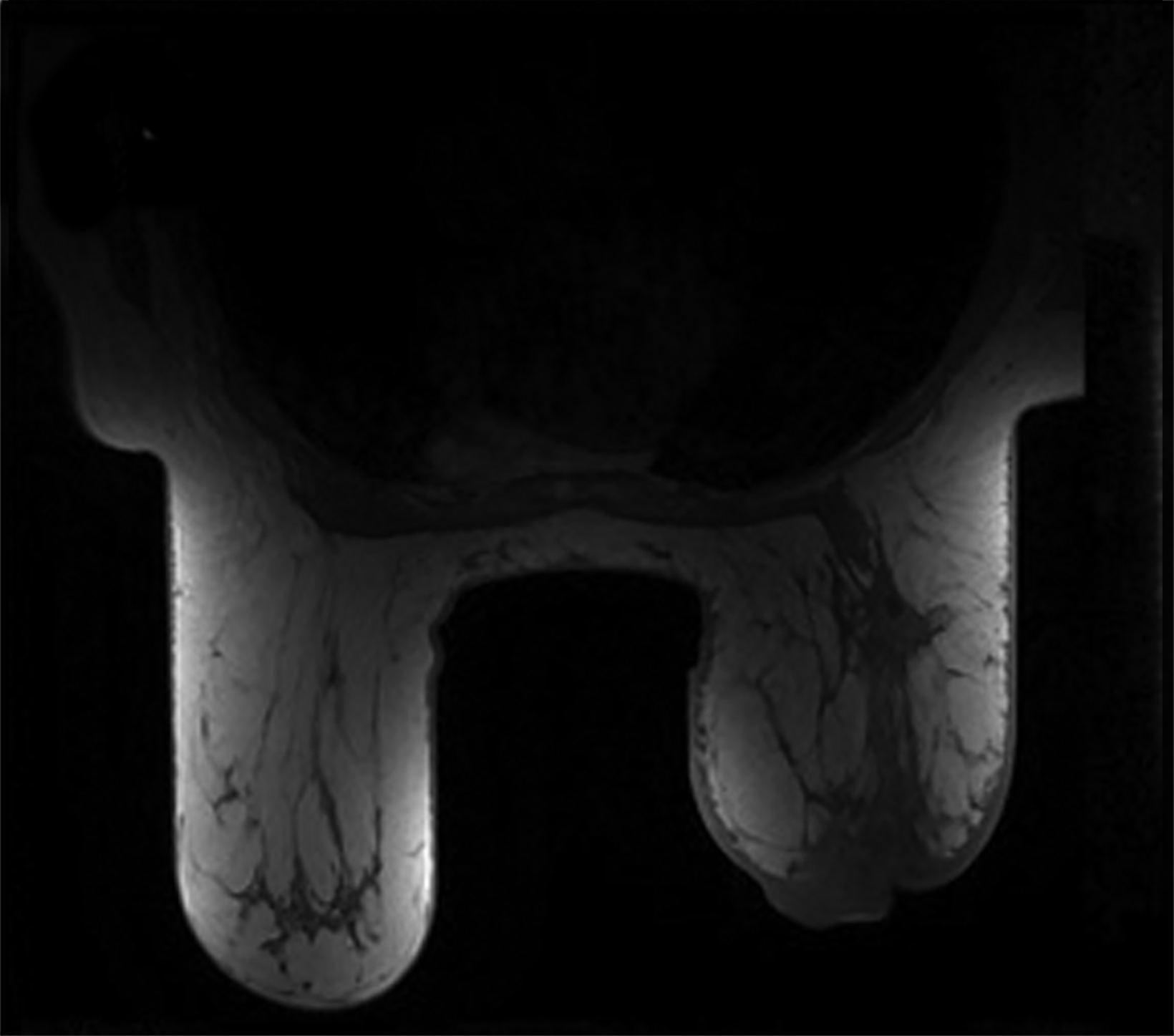
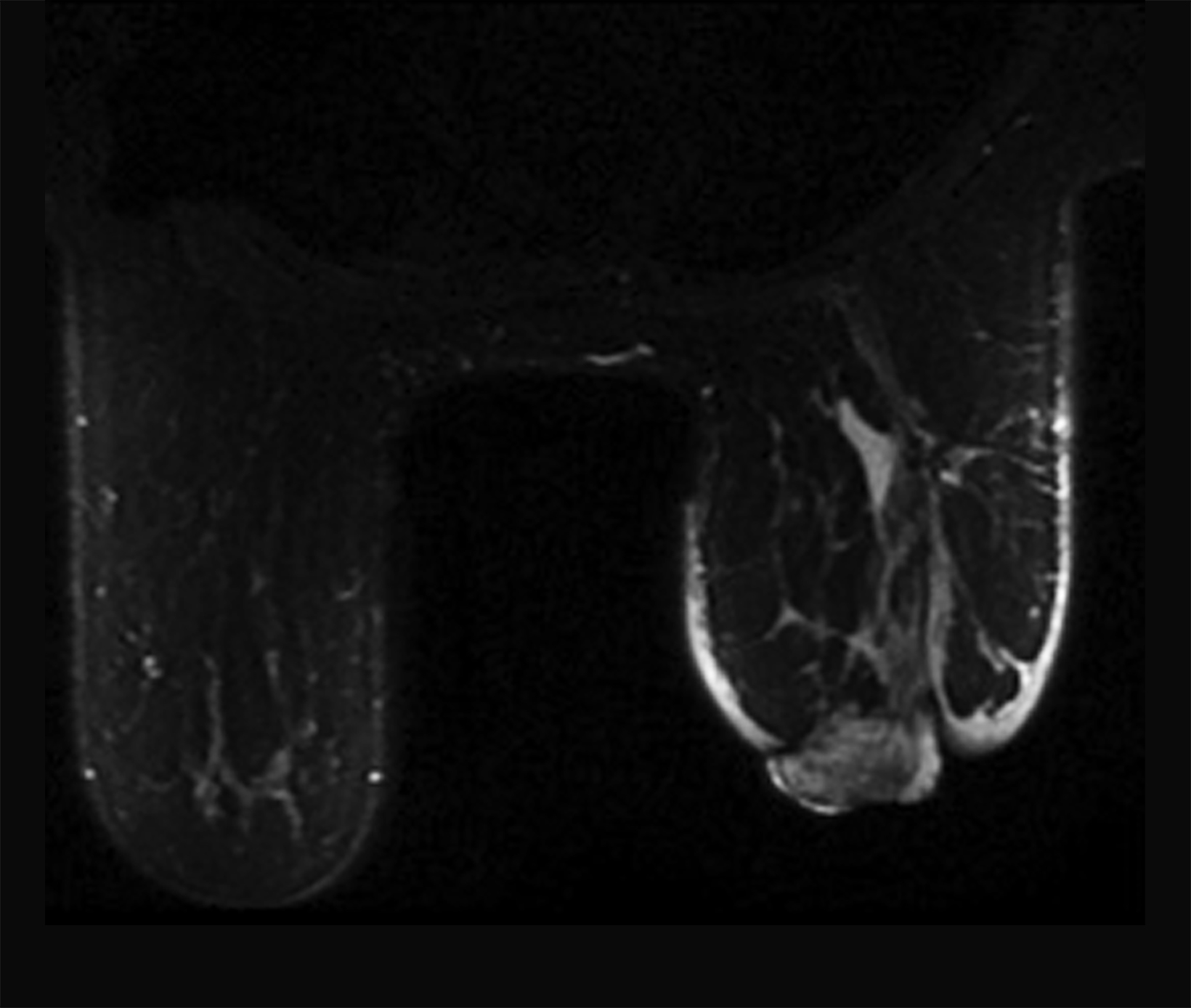
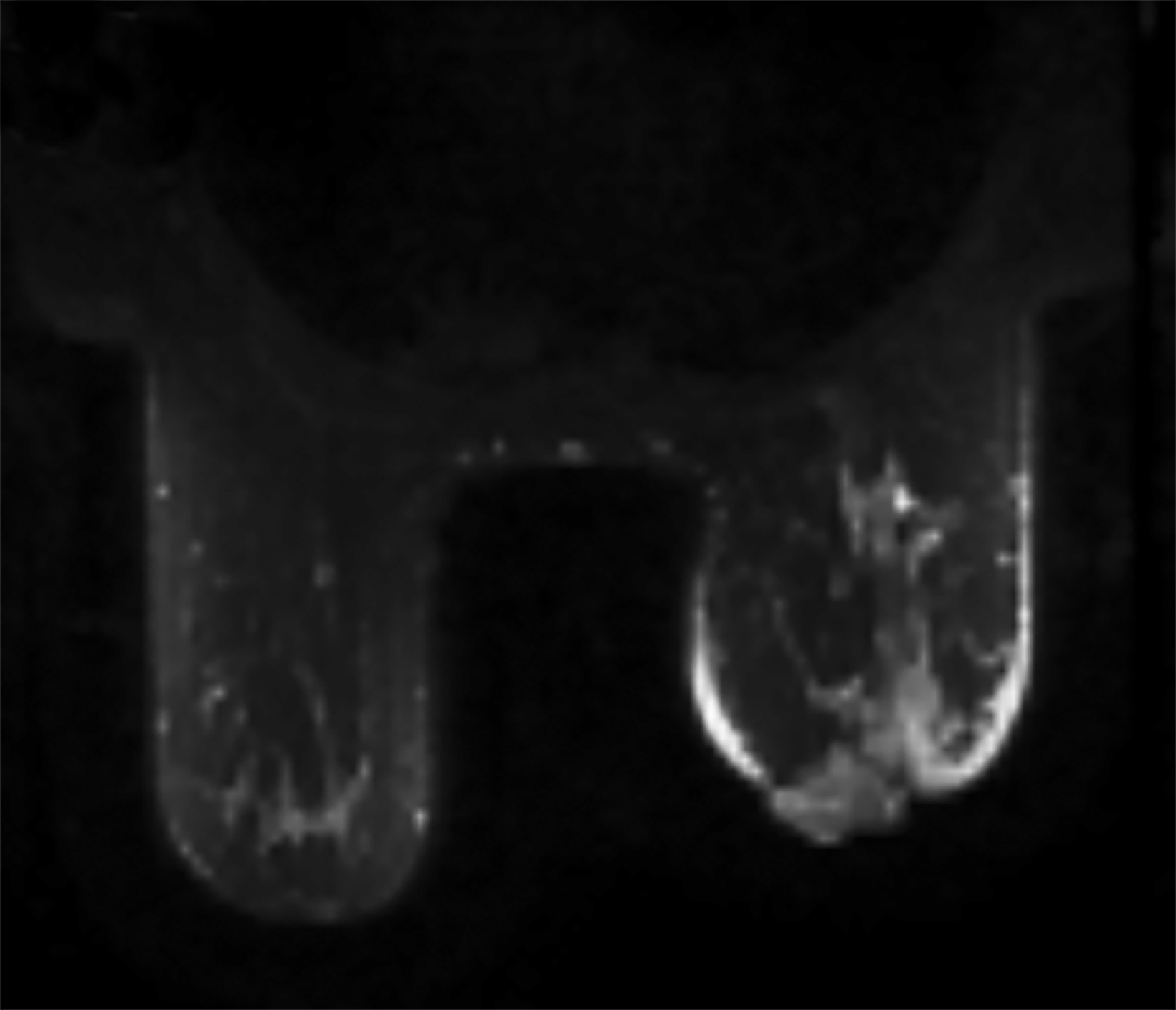
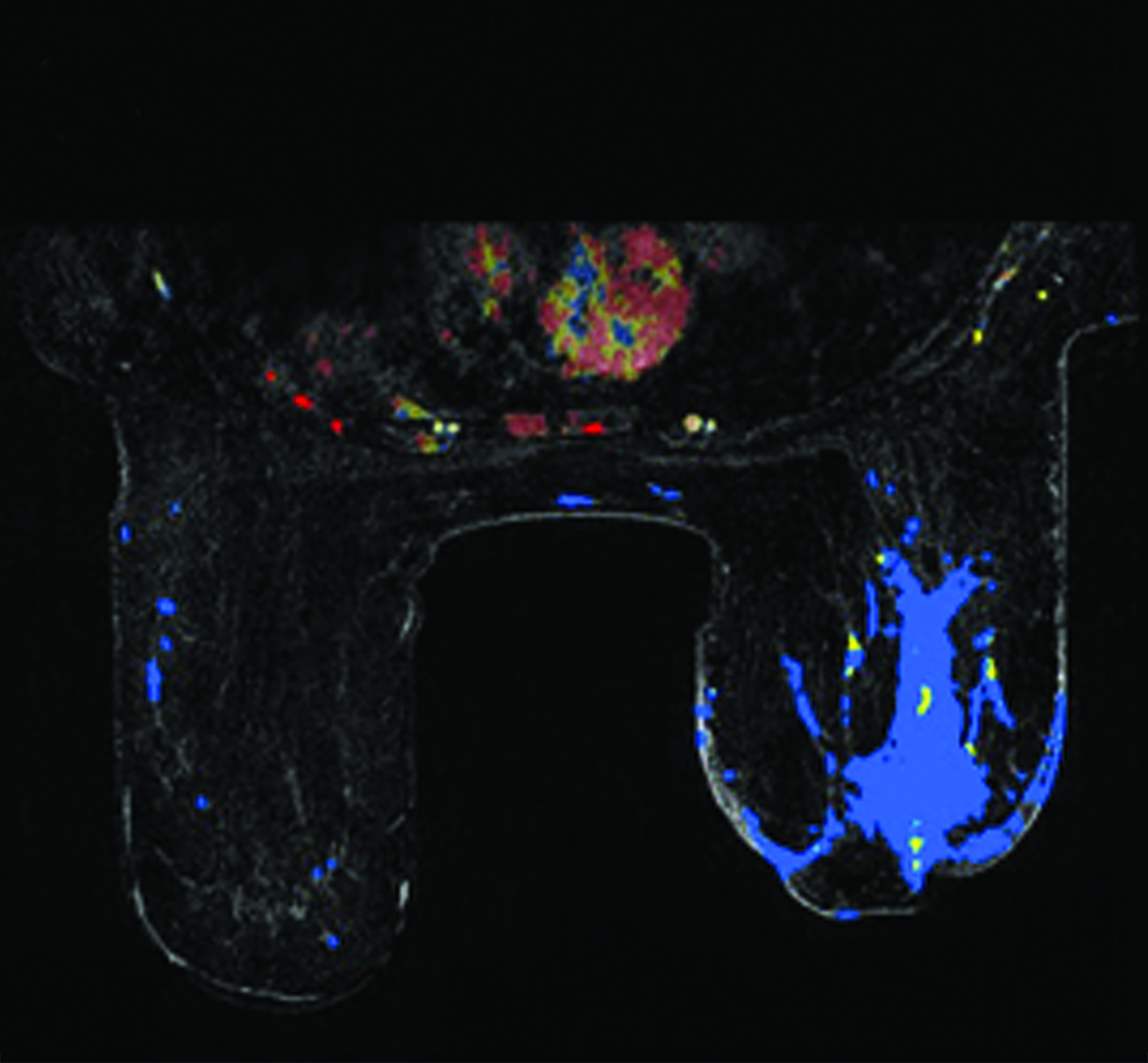
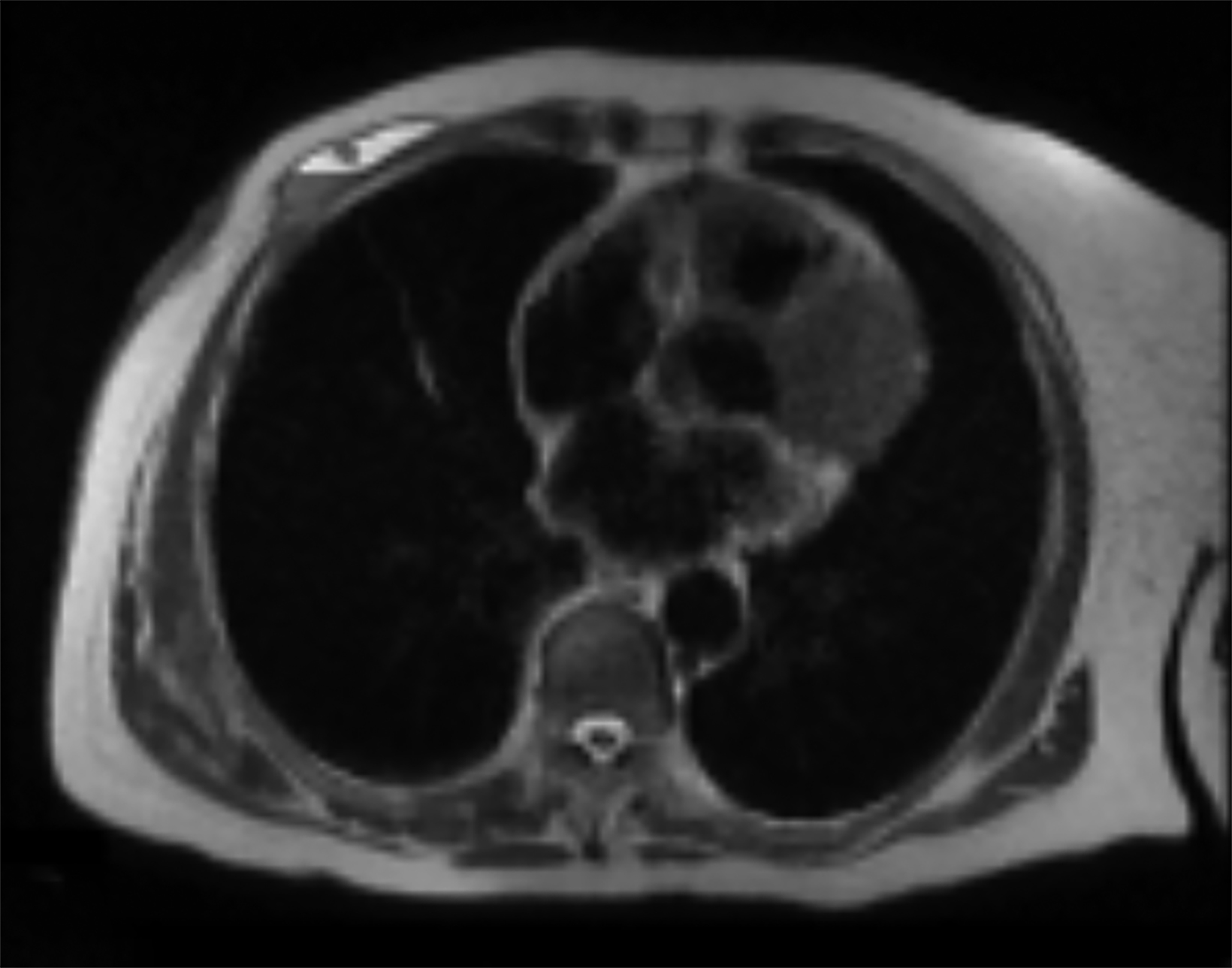
Magnetic resonance imaging (MRI) may be utilized as a surveillance modality in patients at high risk for disease recurrence or development of subsequent disease after radiation therapy. The non-specific findings on ultrasonography and potential false negatives on mammography make MRI an exceptionally valuable imaging modality in the evaluation of RAAS.5 Cutaneous findings, such as skin thickening, skin enhancement, and cutaneous nodules, may be evaluated by MRI. MRI also enables visualization of nipple involvement, parenchymal masses, pectoralis muscle involvement, and lymphadenopathy (Figure 4). Moreover, MRI may assist in identifying residual disease after excisional biopsy and may also guide surgical and treatment planning.24,29
MRI findings of 16 patients with RAAS were reported by Chikarmane et al (2015).22 All patients demonstrated T2 hyperintense skin thickening, while approximately 50% of patients were observed to have discrete lesions with varying intensities and heterogeneity. Four patients had a parenchymal mass, of which all were likewise identified on mammography and 3 were picked up on ultrasonography. Fast initial and washout delayed phase kinetics on contrast enhanced T1 images of cutaneous and intraparenchymal masses were further investigated.22 Sanders et al (2006) described the MRI findings of two cases of angiosarcoma of the breast.30 A nodule in the skin adjacent to a lumpectomy scar with fast initial and plateau delayed phase kinetics was observed in 1 patient while cutaneous enhancement at the lumpectomy scar with fast initial and delayed phase washout kinetics was observed in another patient.30
Differential Diagnosis
Radiologists must be aware that RAAS should be included in the differential diagnosis for any skin changes or parenchymal mass in the setting of prior radiation therapy. Decreased prominence of skin thickening and breast density within 2 years after radiation therapy have been reported to be in keeping with the natural course of breast changes following breast conserving surgery and radiation therapy.24,31-34 Radiologists should be aware of the possibility of recurrent breast carcinoma, inflammatory breast cancer, or mastitis in any patient where an increase in skin thickening or breast density is present years after decreased prominence of these findings. When such findings are accompanied by associated skin discoloration, breast edema, raised skin nodules, papules and/or vesicles, RAAS must be considered in the differential diagnosis.23,24,29 Observable skin changes or parenchymal masses in a quadrant of the breast distinct from the patient’s initial tumour site may further suggest a diagnosis of RAAS.29
RAAS ought to also be differentiated from angiosarcoma associated with chronic lymphedema (also known as Stewart-Treves syndrome). While RAAS occurs in the ipsilateral breast or chest wall thereafter radiation therapy, angiosarcoma associated with chronic lymphedema occurs in the ipsilateral lymphedematous extremity after radical mastectomy for the treatment of breast cancer.5,14
Diagnosing RAAS
Process
Similar to other neoplasms, diagnosis of RAAS is guided by history and physical examination of the patient, which catalyze appropriate imaging and subsequent histologic confirmation of the disease by tissue diagnosis. For tumors which have a propensity for cutaneous involvement, such as RAAS, skin biopsy may be sufficient for diagnosis though deeper lesions require other techniques.35 Though fine needle aspiration (FNA) may identify RAAS, core needle biopsy is preferred because of the risk of false negative results secondary to potential inadequate sample volume for appropriate histologic or immunohistochemical evaluation. Of the patients evaluated by Marchal et al (1999), FNA was found to be rarely conclusive.9 As such, a negative FNA is insufficient to rule out a diagnosis of RAAS in the setting of clinical suspicion for the disease. Should the results of FNA be negative and clinical suspicion for the disease be maintained, core needle biopsy is recommended to obtain an appropriate tissue diagnosis.8,36-38
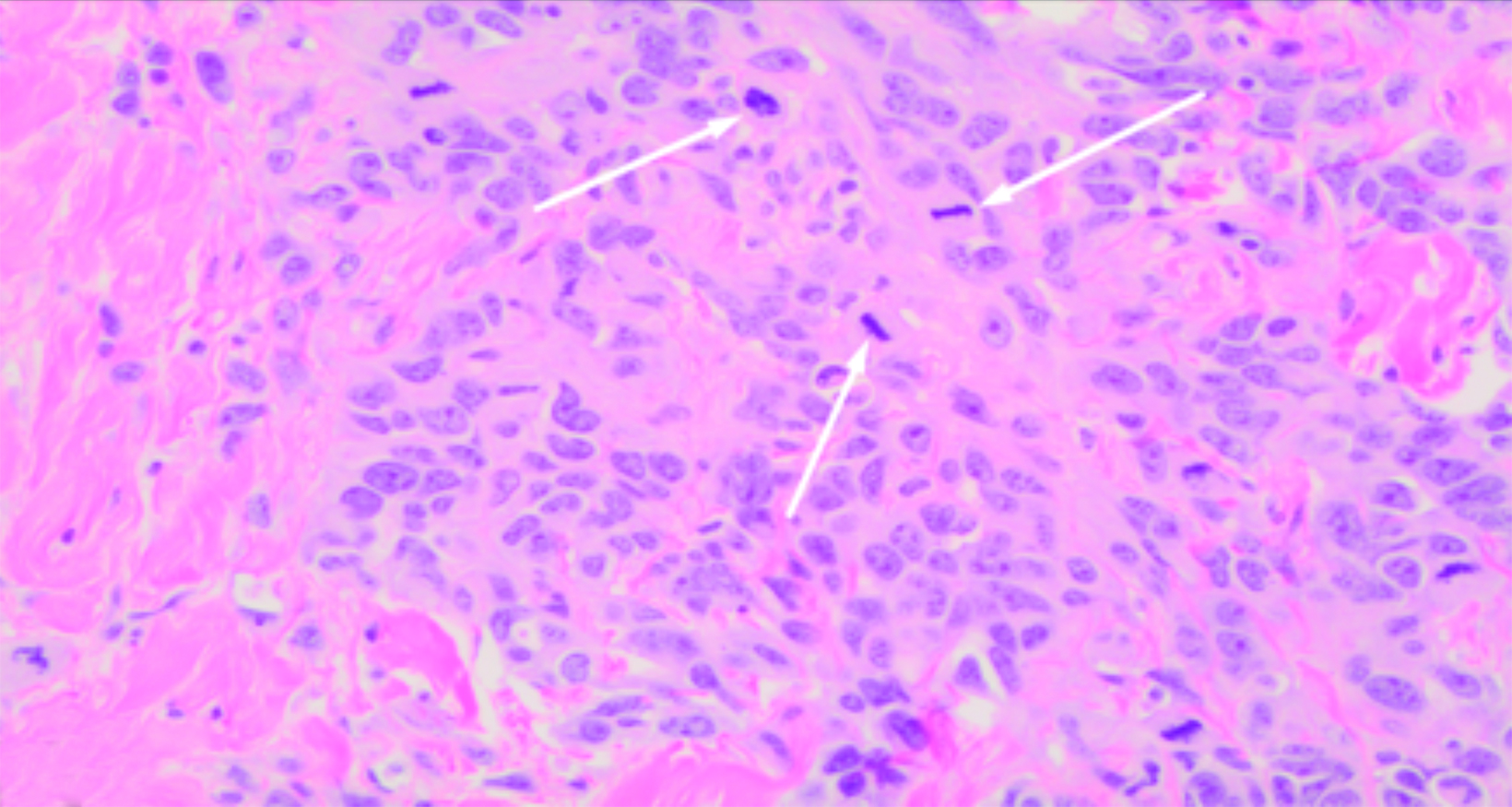
Pathologic and Histologic Features
Tumors may vary significantly in size, with most averaging between 5 to 7 cm. They may be either circumscribed or have infiltrating borders. Areas of hemorrhage may be present. Multifocality is common at the time of presentation and patients may present with extensive involvement of the breast.14
The histologic features of RAAS are similar to those of primary angiosarcoma of the breast.39 However, while nuclear grade usually corresponds to differentiation in primary angiosarcoma of the breast, RAAS generally exhibits poorly differentiated nuclei with vesicular chromatin, prominent nucleoli, and mitotic activity, and it is usually associated with high grade lesions.14
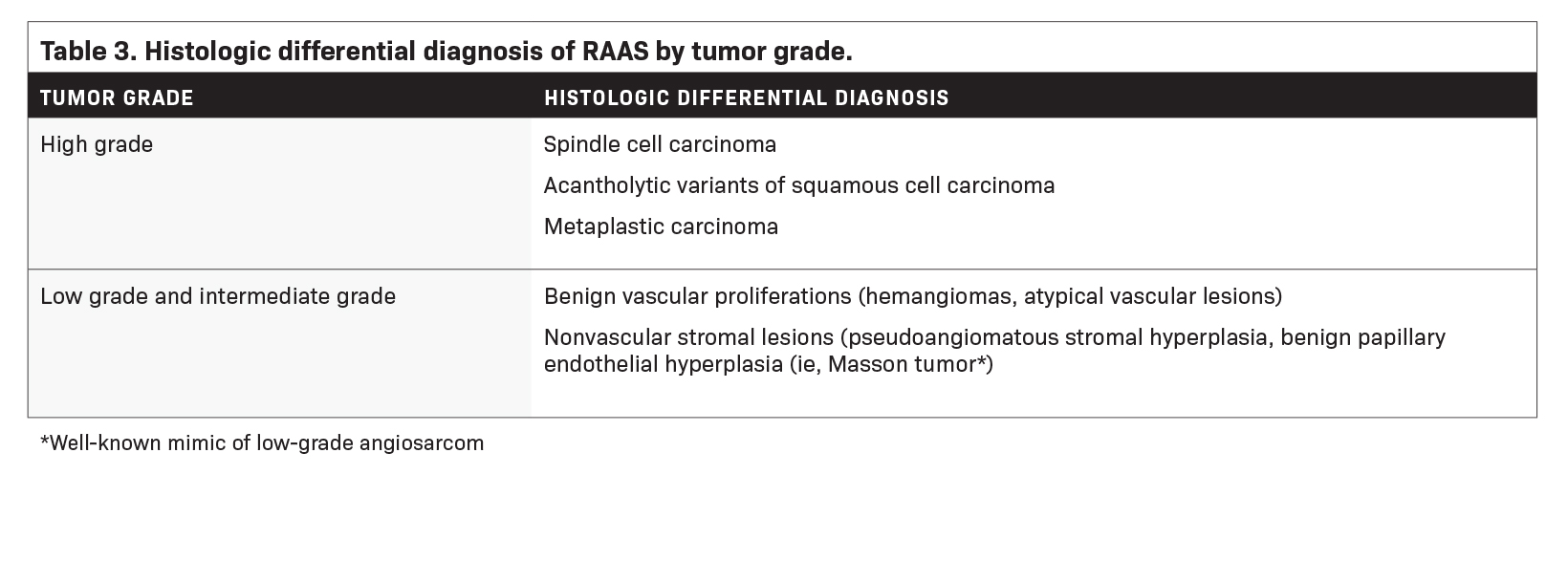
Table 3 summarizes the histologic differential diagnosis of RAAS by tumor grade.14 There is a lack of consensus with respect to the risk of developing angiosarcoma after the diagnosis of atypical vascular lesions.14,40,41 While some reports suggest these lesions to be benign, others have documented either subsequent progression to angiosarcoma or the presence of angiosarcoma within the same region.14,40
Diagnosis of RAAS is confirmed by means of immunohistochemistry staining with the following being expressed: erythroblast transformation specific related gene (transcription factor that confirms vascular lineage), cluster of differentiation 31 (platelet endothelial cell adhesion molecule), cluster of differentiation 34 (human hematopoietic progenitor cell antigen), factor VIII related antigen, Myc protein, and tyrosine kinase receptor KIT (cluster of differentiation 117).20,24,42,43
Staging
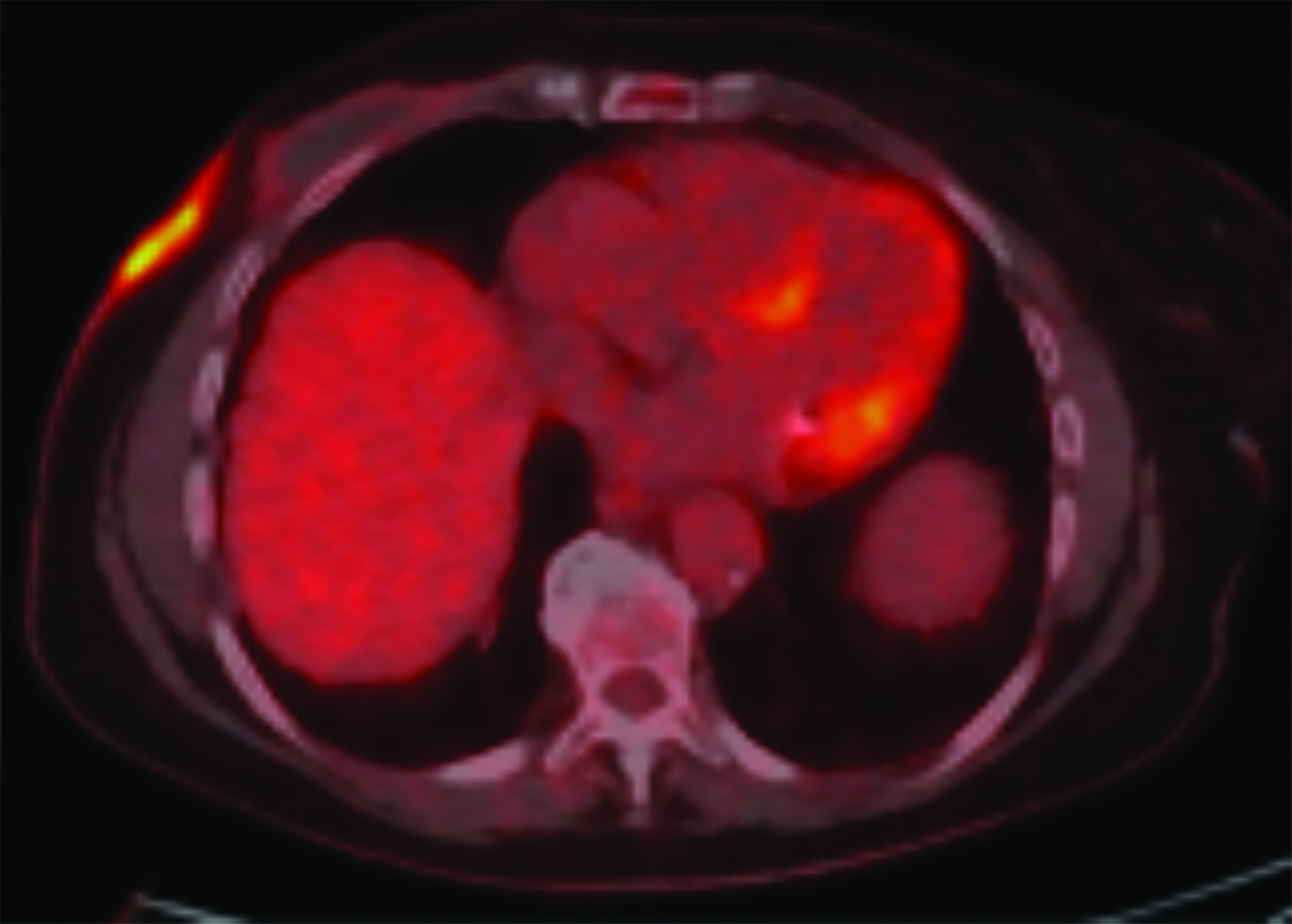
Upon diagnosis of RAAS, staging with positron emission tomography (PET) with fluorodeoxyglucose (18F-FDG) may be completed (Figure 5). Lesions with high maximum standardized uptake values (SUVmax) have been associated with poor prognosis.44
The contralateral breast, chest wall, lungs, liver, and skeleton are frequent sites of metastases.2,20,45 Metastatic disease may be preceded by one or more instances of local recurrence.20
Conclusion
RAAS is a rare but important complication of radiation therapy. With the increased use of breast conserving surgery and radiation therapy in the management of breast cancer, greater awareness and understanding of the disease is required. The clinical presentation of RAAS is variable and appropriate diagnosis is often challenging as skin changes or lesions may be easily mistaken for other entities. Ultrasound may serve as an initial screening modality for the disease but ultrasound findings are nonspecific. Mammographic findings of the disease are likewise nonspecific and may be obscured by expected post-treatment changes after radiation therapy. MRI is superior to ultrasonography and mammography in the evaluation of RAAS. Radiologists must be aware of the disease and its inclusion in the differential diagnosis for any skin changes or breast parenchymal mass that is observed in the setting of prior radiation therapy.
References
- Cormier JN & Pollock RE. Soft tissue sarcomas. CA: Cancer J. 2004;54(2):94-109.
- Glazebrook KN, Magut MJ, Reynolds C. Angiosarcoma of the breast. AJR. 2008;190:533–538.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe. Brit J Radiol. 2017;90:0039.
- Esposito E, Avino F, di Giacomo R, et al. Angiosarcoma of the breast, the unknown-a review of the current literature. Trans Cancer Res. 2019; 8:S510-S517.
- Sheth GR, Cranmer LD, Smith BD, et al. Radiation-induced sarcoma of the breast: a systematic review. The Oncologist. 2012;17(3):405.
- Huang J, Mackillop WJ. Increased risk of soft tissue sarcoma after radiotherapy in women with breast carcinoma. Cancer. 2001;92(1):172–180.
- Mery CM, George S, Bertagnolli MM, et al. Secondary sarcomas after radiotherapy for breast cancer: Sustained risk and poor survival. Cancer. 2009;115:4055–4063.
- Strobbe LJ, Peterse HL, van Tinteren H, et al. Angiosarcoma of the breast after conservation therapy for invasive cancer, the incidence and outcome: an unforeseen sequela. Breast Cancer Res Treat. 1998;47:101–109.
- Marchal C, Weber B, de Lafontan B. Nine breast angiosarcomas after conservative treatment for breast carcinoma: a survey from French comprehensive Cancer Centers. Int J Radiat Oncol Biol Phys. 1999;44:113–119.
- West JG, Quereshi A, West JE, et al. Risk of angiosarcoma following breast conservation: a clinical alert. Breast J. 2005;11:115–123.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499–504.
- Bentley H, Roberts J, Hayes M, et al. The role of imaging in the diagnosis of primary and secondary breast angiosarcoma: twenty-five-year experience of a provincial cancer institution. Clin Breast Cancer. 2022;23(2):e45-e53.
- Scow JS, Reynolds CA, Degnim AC, et al. Primary and secondary angiosarcoma of the breast: the Mayo Clinic Experience. J Surg Onc. 2010;101:401-407.
- Shah S and Marilin R. Radiation-associated angiosarcoma of the breast: clinical and pathologic features. Arch Pathol Lab Med. 2016;140(5):477-481.
- Kelly NP & Siziopikou K. A 68-year-old woman with bluish discoloration of the skin of the breast. Arch Pathol Lab Med. 2002;126(8):989–990.
- Kadouri L, Sagi M, Goldberg Y, et al. Genetic predisposition to radiation induced sarcoma: possible role for BRCA and p53 mutations. Breast Cancer Res Treat. 2013;140(1):207–211.
- Hodgson N, Bowen-Wells C, Moffat F, et al. Angiosarcomas of the breast: A review of 70 cases. Am J Clin Oncol. 2007;30:570–573.
- Vorburger SA, Xing Y, Hunt KK, et al. Angiosarcoma of the breast. Cancer. 2005;104:2682–2688.
- Luini A, Gatti G, Diaz J, et al. Angiosarcoma of the breast: The experience of the European Institute of Oncology and a review of the literature. Breast Cancer Res Treat. 2007;105:81–85.
- Abbott R & Palmieri C. Angiosarcoma of the breast following surgery and radiotherapy for breast cancer. Nat Clin Pract Oncol. 2008;5(12):727-736.
- Bonito FJP, de Almeida Cerejeira D, Dahlstedt-Ferreira C, et al. Radiation-induced angiosarcoma of the breast: A review. Breast J. 2020;26(3):458-463.
- Chikarmane SA, Gombos EC, Jagadeesan J, et al. MRI findings of radiation-associated angiosarcoma of the breast. J Magn Reson Imaging. 2015;42:763–770.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258–260.
- Chesebro AL, Chikarmane SA, Gombos EC, et al. Radiation-associated angiosarcoma of the breast: what the radiologist needs to know. AJR. 2016;207(1):217-225.
- Burns E, Gupta P, Leonard D. Radiation-induced angiosarcoma of the breast. Appl Radiol. 2018;47(3):31A-31C.
- Mermershtain W, Cohen AD, Koretz M, et al. Cutaneous angiosarcoma of breast after lumpectomy, axillary lymph node dissection, and radiotherapy for primary breast carcinoma: Case report and review of the literature. Am J Clin Oncol. 2002;25:597–598.
- Deutsch M & Rosenstein MM. Angiosarcoma of the breast mimicking radiation dermatitis arising after lumpectomy and breast irradiation: A case report. Am J Clin Oncol. 1998;21:608-609.
- Williams EV, Banerjee D, Dallimore N, et al. Angiosarcoma of the breast following radiation therapy. Eur J Surg Oncol. 1999;25:221–222.
- Moore A, Hendon A, Hester M, et al. Secondary angiosarcoma of the breast: can imaging findings aid in the diagnosis? Breast J. 2008;14:293–298.
- Sanders LM, Groves AC, Schaefer S. Cutaneous angiosarcoma of the breast on MRI. AJR. 2006;188:W143–W146.
- Dershaw DD, Shank B, Reisinger S. Mammographic findings after breast cancer treatment with local excision and definitive irradiation. Radiology. 1987;164:455–461.
- Peters ME, Fagerholm MI, Scanlan KA, et al. Mammographic evaluation of the postsurgical and irradiated breast. RadioGraphics. 1988;8:873–899.
- Harris KM, Costa-Greco MA, Baratz AB, et al. The mammographic features of the postlumpectomy, postirradiation breast. RadioGraphics. 1989;9:253–268.
- Chansakul T, Lai KC, Slanetz PJ. The postconservation breast: part 2, imaging findings of tumor recurrence and other long-term sequelae. AJR. 2012;198:331–343.
- Vesoulis Z & Cunliffe C. Fine-needle aspiration biopsy of post-radiation epithelioid angiosarcoma of breast. Diagn Cytopathol. 2000;22:172–175.
- Sessions SC & Smink RD. Cutaneous angiosarcoma of the breast after segmental mastectomy and radiation therapy. Arch Surg. 1992;127:1362–1363.
- Majeski J, Austin RM, Fitzgerald RH. Cutaneous angiosarcoma in an irradiated breast after breast conservation therapy for cancer: Association with chronic breast lymphedema. J Surg Oncol. 2000;74:208–213.
- Gherardi G, Rossi S, Perrone S, et al. Angiosarcoma after breast-conserving therapy: Fine-needle aspiration biopsy, immunocytochemistry, and clinico-pathologic correlates. Cancer. 2005;105:145–151.
- Lucas DR. Angiosarcoma, radiation-associated angiosarcoma, and atypical vascular lesion. Arch Pathol Lab Med. 2009;133(11):1804–1809.
- Patton KT, Deyrup AT, Weiss SW. Atypical vascular lesions after surgery and radiation of the breast: a clinicopathologic study of 32 cases analyzing histologic heterogeneity and association with angiosarcoma. Am J Surg Pathol. 2008;32(6):943-950.
- Fraga-Guedes C, Gobbi H, Mastropasqua MG, et al. Clinicopathological and immunohistochemical study of 30 cases of post-radiation atypical vascular lesion of the breast. Breast Cancer Res Treat. 2014;146(2):347–354.
- Brenn T & Fletcher CD. Postradiation vascular proliferations: an increasing problem. Histopathology. 2006;48:106–114.
- Morgan EA, Kozono DE, Wang Q, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol. 2012;19:3801–3808.
- Cassou-Mounat T, Champion L, Bozec L. Primary and secondary breast angiosarcoma: FDG PET/CT series. Clin Nucl Med. 2019;44(1):e33-e35.
- Billings SD, McKenney JK, Folpe AL, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation: an analysis of 27 cases. Am J Surg Pathol. 2004;28:781–788.
- Cahan WG & Woodard HQ. Sarcoma arising in irradiated bone; report of 11 cases. Cancer. 1948;1:3–29.
- Arlen M, Higinbotham NL, Huvos AG. Radiation-induced sarcoma of bone. Cancer. 1971;28:1087–1099.
Citation
H B, J Y, M H, T M. Radiation-associated Angiosarcoma of the Breast: A Clinicopathological and Multimodality Imaging Review. Supplement to Applied Radiology. 2024;(1):26-35.
January 25, 2024