Prostate Cancer Patient Outcomes Improve With Personalized Dosing
Images
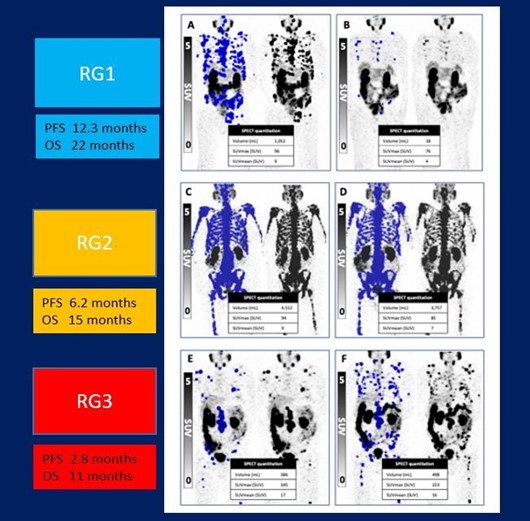
Physicians can personalize dosing intervals, significantly improving patient outcomes, by monitoring early-response biomarkers in men undergoing 177Lu-PSMA prostate cancer treatment. In a study presented at the Society of Nuclear Medicine and Molecular Imaging 2023 Annual Meeting, early stratification with 177Lu-SPECT/CT allowed men responding to treatment to take a “treatment holiday” and allowed those not responding the option to switch to another treatment.
Approved by the U.S. Food and Drug Administration in 2022, 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer. However, not all men respond equally to treatment, with some responding very well and others progressing early.
“Currently, a standardized dosing interval is used for 177Lu-PSMA treatment,” said Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the Department of Theranostics and Nuclear Medicine at St. Vincent's Hospital in Sydney, Australia. “However, monitoring early-response biomarkers to adjust treatment intervals may improve patient outcomes.”
In the study, researchers sought to evaluate progression-free survival and overall survival of different dosing intervals. Study participants included 125 men who were treated in a clinical program with six weekly doses of 177Lu-PSMA. The men were imaged with 177Lu-SPECT/CT after each dose. After the second dose, researchers analyzed the men’s prostate specific antigen (PSA) levels and the 177Lu-SPECT response to determine ongoing management.
Patients were grouped by level of response. Those in Response Group 1 (35% of participants) had a marked reduction in PSA level and partial response on 177Lu-SPECT and were advised to cease treatment until PSA levels rose. Response Group 2 (34%) saw stable or reduced PSA and stable disease on SPECT imaging; these men continued on their six week treatment plan until no longer clinically beneficial. In Response Group 3 (31%), men saw a rise in PSA levels and had progressive disease on SPECT imaging. These patients were offered the opportunity to try a different treatment.
PSA levels decreased by more than 50% in 60% of patients. Overall study participants had a median PSA progression-free survival of 6.1 months and a median overall survival of 16.8 months. Median PSA progression-free survival was 12.1 months, 6.1 months, and 2.6 months, for Response Groups 1, 2 and 3, respectively. The overall survival was 19.2 months for Response Group 1, 13.2 months for Response Group 2, and 11. 2 months for Response Group 3. Additionally, for those in Response Group 1 who had a “treatment holiday,” the median treatment-free time was 6.1 months.
“Personalized dosing allowed one-third of the men in this study to have treatment breaks while still achieving the same progression-free and overall survival outcomes they would have if they received continuous treatment,” noted Nguyen. “It also allowed another one-third of men who had early biomarkers of disease progression the opportunity to try a more effective potential therapy if one was available.”
Patients at St. Vincent's Hospital will continue to be stratified by these early response biomarkers. Once validated in a prospective clinical trial, Nguyen hopes that this stratification strategy will become more widely available for patients.