Pediatric Cephaloceles: A Multimodality Review
Images
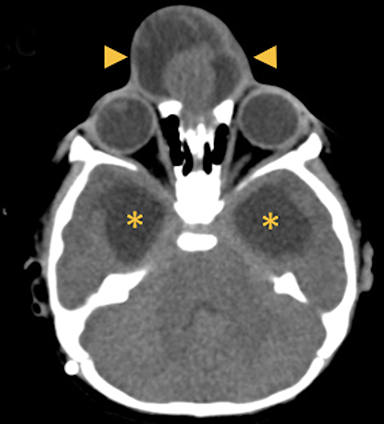
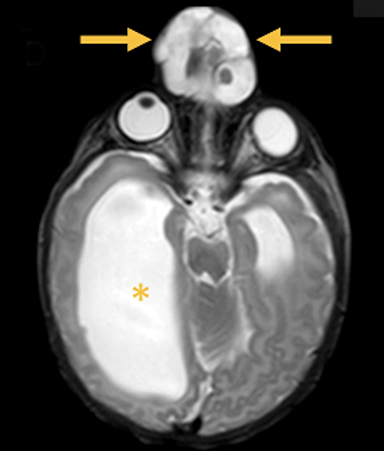
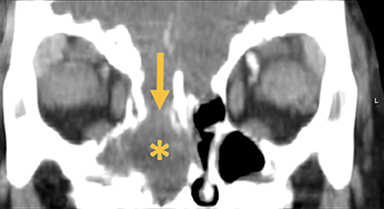
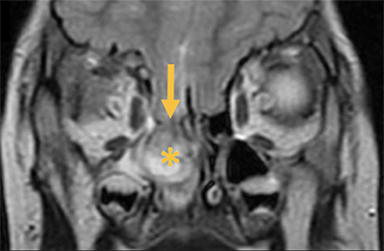
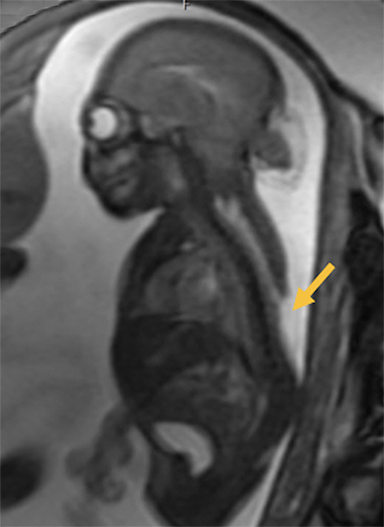
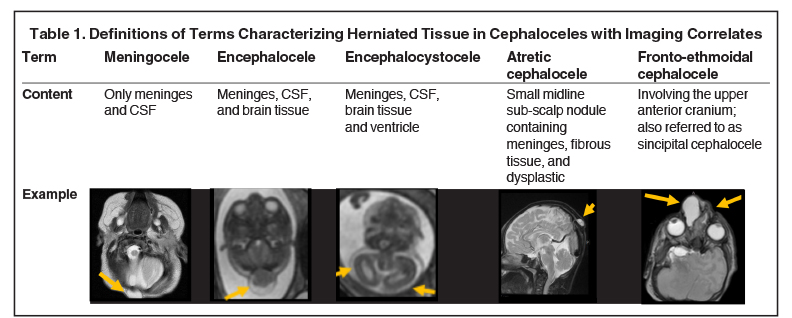
Cephaloceles are complex brain malformations that can be further characterized by the content of the herniated tissue. They can be classified by location, which is important for family counseling and surgical planning. Although imaging is vital for characteriz ing cephaloceles, and fetal MRI is becoming more commonly utilized for prenatal characterization, little appears in the radiology literature on this complex topic. This review illustrates the four major types of cephaloceles using a multimodality approach with prenatal and postnatal correlation. A brief overview of epidemiology and embryology is provided, and associated anomalies and distinguishing features of associated syndromes are discussed. By utilizing the location-based system and understanding the commonly associated features, the radiologist may provide a more comprehensive description of cephaloceles to better facilitate clinical management.
Cephaloceles are one of the most common forms of neural tube defects, ranking only behind myelomeningoceles and anencephaly. Cephalocele is a generic term defined as a protrusion of the meninges with or without brain tissue through a defect in the skull.1-2 A meningocele is a protrusion of only meninges and cerebrospinal fluid (CSF). An encephalocele is a protrusion of meninges, CSF, and brain tissue. An encephalocystocele contains meninges, CSF, brain tissue and ventricle. The term atretic cephalocele (also called meningocele manqué) describes a small, midline subscalp nodule that contains meninges, fibrous tissue, and dysplastic brain tissue.3 The term fronto-ethmoidal denotes the involvement of the upper anterior cranium and is synonymous with the less commonly used sincipital (Table 1).
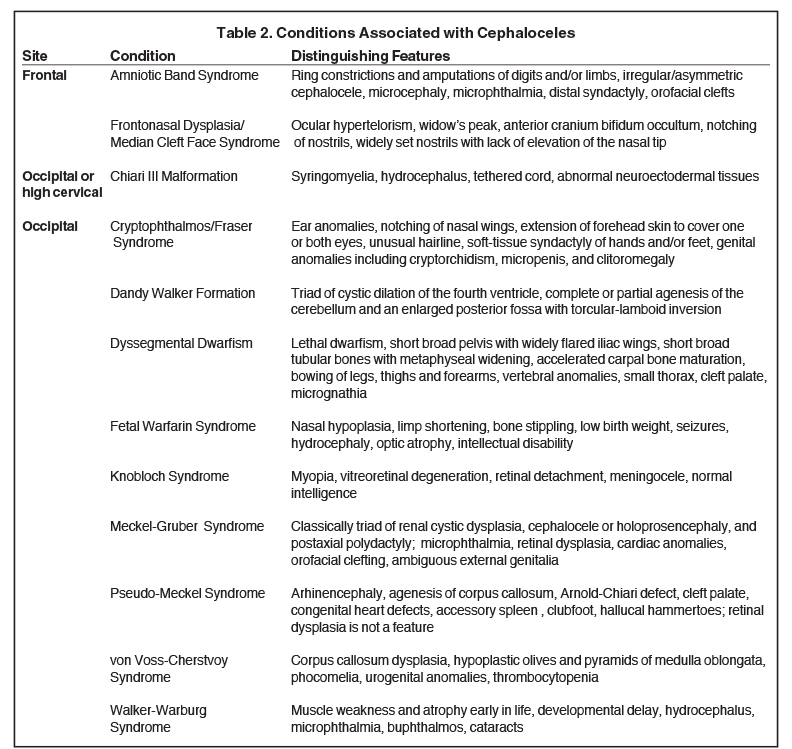
The incidence of cephaloceles is 0.8-4 of 10,000 live births.4 Cephaloceles account for 10-20% of all craniospinal dysraphisms.4 Geographical variation exists, with occipital subtypes representing 66-89% of all cephaloceles in the Caucasian populations of North America and Western Europe.5-7 Anterior subtypes are more common in Southeast Asia.6 Most cases of isolated cephaloceles (not associated with other congenital anomalies) are sporadic, with genetic and non-genetic factors involved in the pathogenesis.8 Cephaloceles may be associated with myriad genetic syndromes, most commonly Meckel-Gruber, as well as the Chiari III malformation, holoprosencephaly, and Dandy-Walker malformation.2,9-10 A cephalocele detected prenatally warrants a detailed diagnostic assessment and characterization for an underlying syndrome.11
Embryology
The central nervous system begins forming in the third week of embryonic life as thickened ectoderm called the neural plate. Elevation of the lateral edges of the neural plate forms the neural folds, which fuse to form the neural tube. Fusion begins in the cervical region, proceeding in both the rostral and caudal directions until closure between days 25-27 postconception. The mechanism resulting in cephaloceles is uncertain but presumably involves defective closure of the anterior neural tube. Anterior cephaloceles (fronto-ethmoidal and basal) are thought to arise from defective development of the prosencephalic neural crest tissue.12 In contrast, occipital cephaloceles may relate to defective segmentation of the posterior cranial bones.13 Some authors believe the etiology of congenital cephaloceles centers on a postneurulation event in which brain tissue herniates through a defect in the mesenchyme that eventually becomes the cranium and dura.14
Diagnosis and Classification
Most cephaloceles can be detected prenatally by ultrasound. Alpha fetoprotein levels may be unreliable given that both maternal serum and amniotic fluid alpha fetoprotein levels may be normal if the cephalocele is covered by skin.15 On ultrasound these lesions may appear as cystic (meningocele) and/or solid (encephalocele) structures protruding through a calvarial defect. Further evaluation with computed tomography (CT) and magnetic resonance imaging (MRI, including fetal MRI) is useful to determine the extent of herniation as well as the presence of associated anomalies. CT is useful for identifying osseous defects while MRI is superior for defining the portions of herniated tissue, detecting abnormal signal in dysplastic brain tissue, and evaluating the cartilaginous nasofrontal region. An MR or CT angiogram or venogram may provide more detailed evaluation of vascular anatomy and its relation to the cephalocele.
Several classification systems exist. The system proposed by Suwanwela and Suwanwela provides a comprehensive, location-based classification that characterizes cephaloceles as 1) occipital, 2) cranial vault, 3) fronto-ethmoidal, and 4) basal. This system has also been found useful for selecting the operative approach.6 More extensive cephaloceles, however, may encompass more than one type. Clinical features and prognoses of cephaloceles depend on location, severity, and presence of dysplastic brain tissue and associated abnormalities.16-17
Occipital Cephaloceles
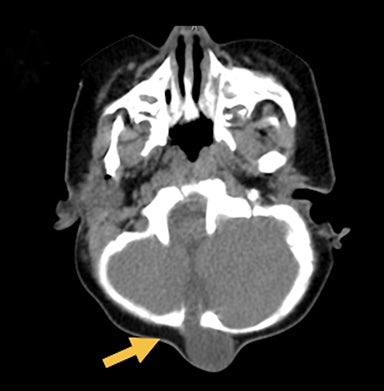
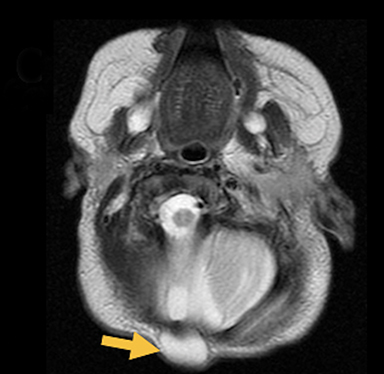
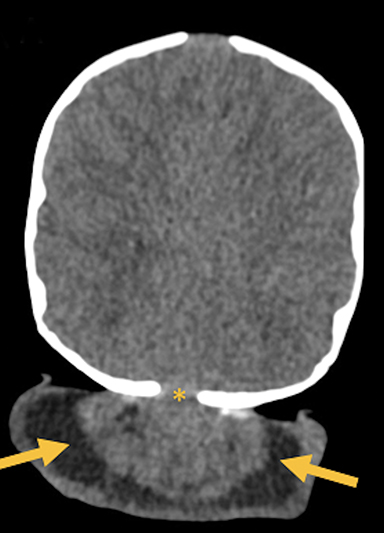
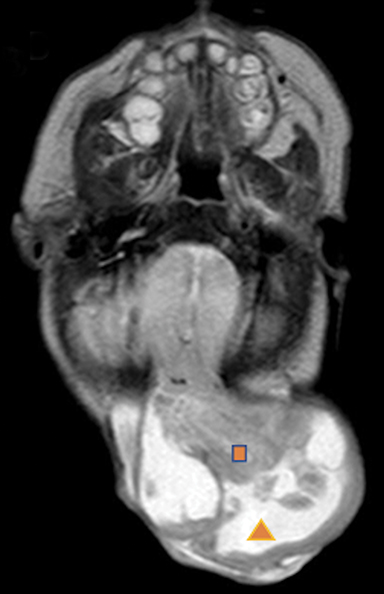
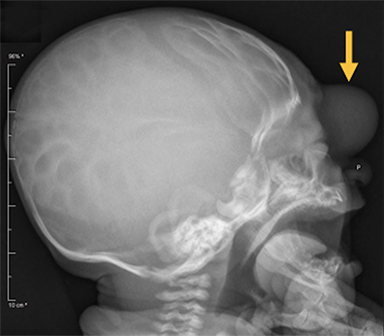
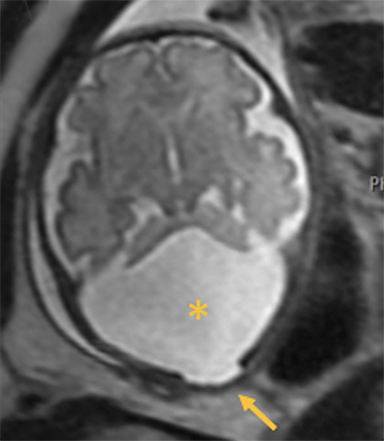
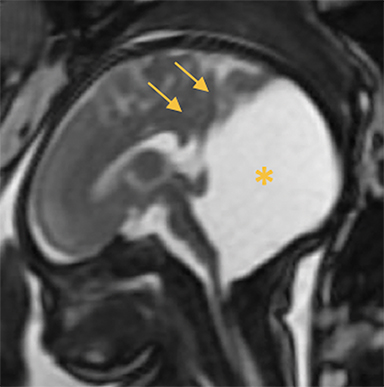
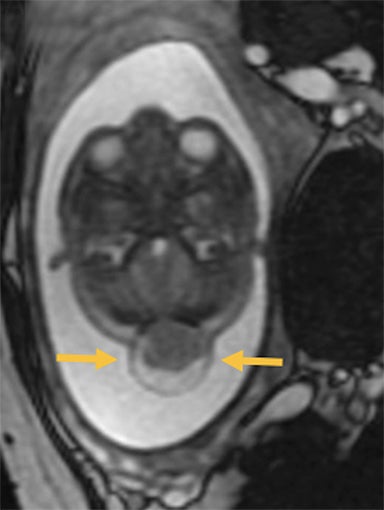
Occipital cephaloceles demonstrate defects involving the occipital bones, with the cephalocele extending posteriorly (Figures 1-3). The herniated tissue may include the supra- and/or infratentorial brain, tentorium, and dural venous sinuses. These cephaloceles are the most common type overall and account for a higher proportion of cephaloceles in Caucasian populations of Europe and North America.5-7 Occipital cephaloceles are typically apparent on physical exam at birth, and the size of the herniation varies. Ventriculomegaly is observed in the majority of cases. Prognostic factors include size of herniation, degree of hydrocephalus, and presence of associated anomalies.16-17 Large occipital encephaloceles may be associated with developmental delay, blindness, poor feeding, cranial nerve deficits, and seizures.18
Cranial Vault Cephaloceles
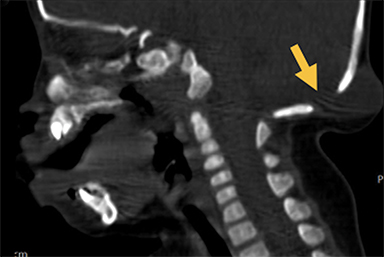
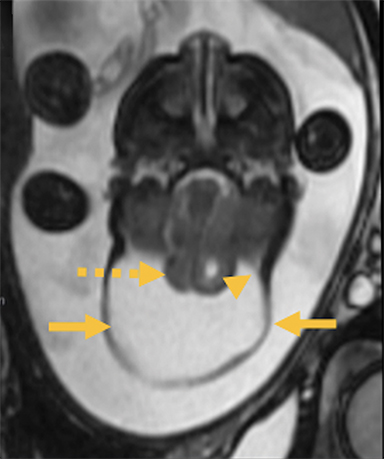
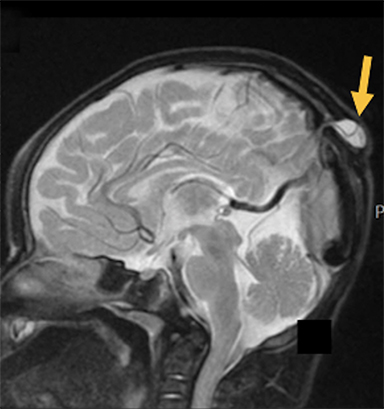
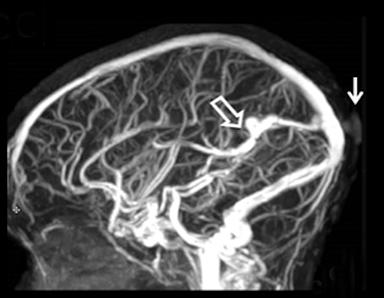
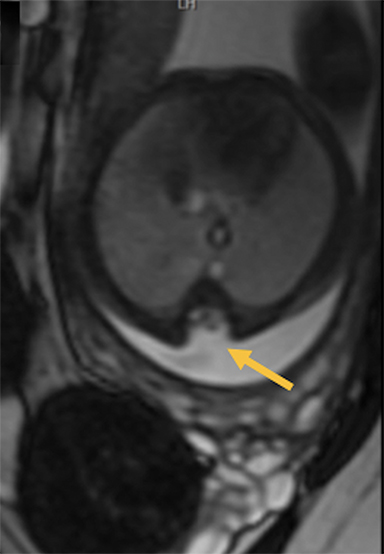
Cranial vault cephalocele occur along the superior cranium within the fontanelles or defects in the parietal, frontal, or temporal bones. They present as a midline posterior scalp mass. The patient often is otherwise clinically normal unless associated anomalies are present.19 Atretic cephaloceles, the most common form, are small midline subcutaneous scalp masses consisting of dura and dysplastic meninges connected to the intracranial meninges by a fibrous stalk. They are usually located in the parietal lobe; MRI typically demonstrates a fibrous tract and vertical falcine vein, which extend to a subcutaneous scalp mass (Figure 4). Atretic cephalocele may arise through a bone defect or fenestration, or the bone may be closed with completely separated intra- and extracranial contents.20
The embryonic falcine sinus is often positioned vertically, with a cigar-shaped CSF tract in the interhemispheric fissure.19,21 Cranial vault cephaloceles are considered abortive or involuted true cephaloceles20 ; they have a more favorable prognosis than other true cephaloceles.22
Fronto-ethmoidal Cephaloceles
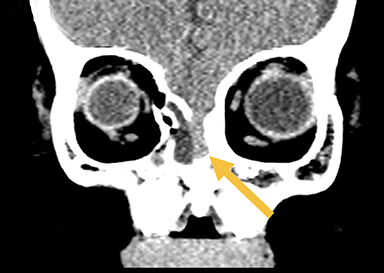
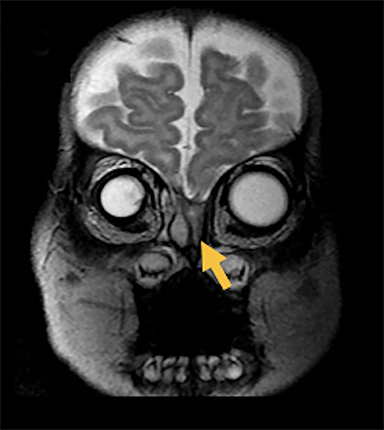
Fronto-ethmoidal cephaloceles (FECs) range from occult lesions to marked craniofacial abnormalities, including microcephaly, telecanthus, hypertelorism, orbital dystopia, or micro/anophthalmos. There is an increased incidence in Southeast Asian populations.6 Sagittal and coronal images may be the most helpful in demonstrating contiguity between intracranial contents and the mass.23 Prior to surgical repair, CT scanning helps to characterize the bone defect. FECs can be classified according to the osseous defect’s location.
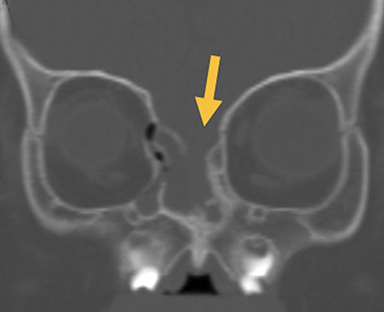
Naso-ethmoidal cephaloceles are characterized by herniation into the superomedial nasal cavity, with the defect centered at the foramen cecum (Figure 5).6 They protrude through the foramen cecum into the prenasal space. They are positioned inferior to the nasal bones.
Naso-frontal cephaloceles have a midline frontal defect, often with mass at the glabella (root of the nose) (Figure 6).6 They protrude through an unobliterated fonticulus frontalis.
Naso-orbital cephaloceles are characterized by an inferomedial orbital defect. They protrude into the inferomedial orbit through a defect in the maxillary bones at the lacrimal/frontal process. They can induce proptosis and globe displacement.
FECs are more commonly associated with craniofacial clefts.6 FECs also generally have a better prognosis than occipital cephaloceles because the protruding mass in the FEC tends to contain scarred, nonfunctional neural tissue.18
Basal Cephaloceles
Basal cephaloceles occur when there is a defect in skull base (Figure 7). They are rare and may present even later in the first decade of life with recurrent meningitis.24 Basal cephaloceles may be occult or present with midface anomalies such as cleft lip/plate, hypertelorism, or a nasal epipharyngeal mass. Immediate surgical repair is indicated because of the elevated risk of meningitis.18
Associated Anomalies and Neurologic Manifestations
Many cases of cephalocele are associated with additional congenital anomalies, which are important to identify for prognostic purposes (Table 2). In one of the largest studies on cephaloceles, the following associated anomalies and neurologic manifestations were identified in order of decreasing frequency: hydrocephalus, seizure disorder, corpus callosum abnormalities, cerebral dysgenesis, and migrational disorders, including gray matter heterotopia, microcephaly, and myelomeningocele.4
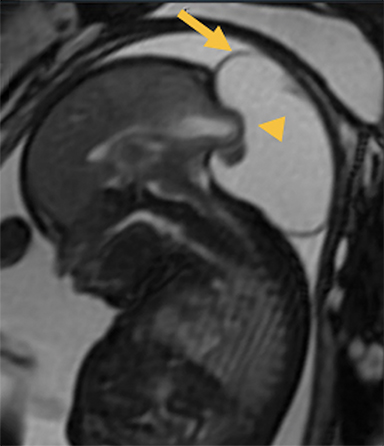
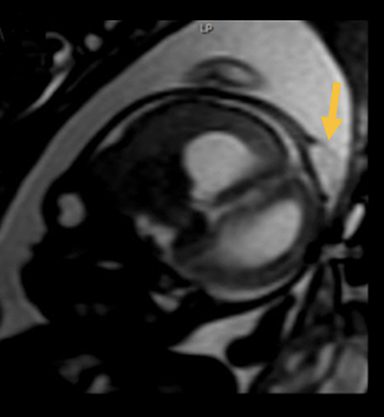
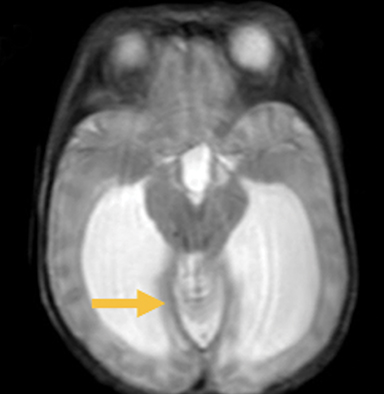
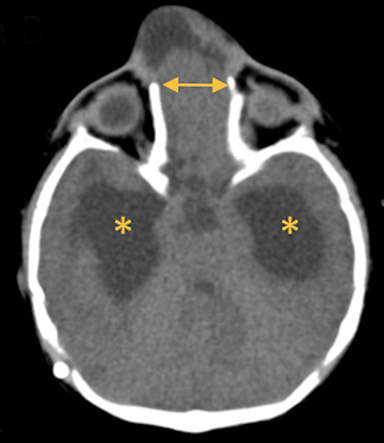
In this study, 52% of patients experienced at least mild developmental delay, with hydrocephalus and other associated intracranial abnormalities identified as predictors of developmental delay.4 Lesion location notably was not found to be a significant predictor of outcome. Myriad genetic syndromes and conditions are associated with cephaloceles, including Meckel-Gruber syndrome, the middle interhemispheric variant of holoprosencephaly, Dandy-Walker malformation (Figure 8) and Chiari III malformation (Figure 9).2,9-10 Meckel Gruber is the most commonly associated syndrome.25 With the presence of associated malformations affecting cognitive outcome, imaging is a critical component of the workup of patients with cephaloceles and often serves as the primary basis for prenatal counseling.4
Conclusion
Cephaloceles are complex cranial malformations that can be classified by location, each with differing clinical presentation and associated anomalies. Prenatal and postnatal imaging is important to delineate the relevant anatomy. By describing cephaloceles via a location-based classification system, the radiologist may facilitate more accurate presurgical planning and prenatal counseling.
References
- Diebler C, Dulac O. Cephaloceles: Clinical and neuroradiological appearance. Neuroradiology. 1983; 25:199-216.
- Naidich TP, Altman NR, Braffman BH, et al. Cephaloceles and related malformations. AJNR Am J Neuroradiol. 1992;13: 655-690.
- Yokota A, Kajiware H, Kohchi M. Parietal cephalocele: Clinical importance of its atretic form and associated malformations. J Neurosurg. 1988; 69:545-551.
- Lo BW, Kulkarni AV, Rutka JT, et al. Clinical predictors of developmental outcome in patients with cephaloceles. J Neurosurg Pediatr. 2008; 2(4):254-257.
- Simpson DA, David DJ, White J. Cephaloceles: Treatment, outcome and antenatal diagnosis. Neurosurgery. 1984; 15:14-21.
- Suwanwela C, Suwanwela N. A morphological classification of sincipital encephalomeningoceles. J Neurosurg. 1972:36(2):201-211.
- Chapman PH, Swearingen B, Caviness VS. Subtorcular occipital encephaloceles: Anatomical considerations relevant to operative management. J Neurosurg. 1989; 71:375-381.
- Copp AJ, Stanier P, Greene ND. Neural tube defects: Recent advances, unsolved questions, and controversies. Lancet Neurol. 2013; 12(8):799-810.
- Cohen MM. Mutations affecting craniofacial cartilage. Biomedical aspects. New York: Academic Press 1983(53):191-228.
- Cohen MM, Lemire RM. Syndromes with cephaloceles. Teratology.1982; 25:161-172.
- Thompson DN. Postnatal management and outcome for neural tube defects including spina bifida and encephaloceles. Prenat Diagn. 2009; 29:412-419.
- Hoving EW, Vermeij-Keers C. Frontoethmoidal encephaloceles, a study of their pathogenesis. Pediatr Neurosurg. 1997; 27(5): 246-256.
- Tavella S, Bobola N. Expressing Hoxa2 across the entire endochondral skeleton alters the shape of the skeletal template in a spatially restricted fashion. Differentiation. 2010; 79(3):194-202.
- Gluckman TJ, George TM, McLone DG. Postneurulation rapid brain growth represents a critical time for encephalocele formation: a chick model. Pediatr Neurosurg. 1996; 25;130-136.
- Sabbagha RE, Tamura RK, Dal Compo S, et al. Am J Obstet Gynecol. 1980; 138(5): 511-517.
- Raja RA, Qureshi AA, Memon AR, et al. Pattern of encephaloceles: a case series. J Ayub Med Coll Abbottabad. 2008; 20:125-128.
- Kiymaz N, Yilmaz N, Demir I, et al. Prognostic factors in patients with occipital encephalocele. Pediatr Neurosurg. 2010; 46:6-11.
- Chern JJ, Bollo RJ, Governale LS, et. al. Operative Neurosurg. 2019; 17(1) Supplement: S182-S208.
- Patterson RJ, Egelhoff JC, Crone KR, et al. Atretic parietal cephaloceles revisited: An enlarging clinical and imaging spectrum? AJNR Am J Neuroradiol. 1998;19(4):791-795.
- Favoreel N, Devooghdt M, Devlies F, et al. Atretic cephalocele. J Belgian Soc. Radiol. 2015: 98(3):119–120.
- Murakami N, Morioka T, Kawamura N, et al. Venous anomaly analogous to vertical embryonic positioning of the straight sinus associated with atretic cephalocele at the suboccipital region. Childs Nerv Syst. 2017:33(1):179-182.
- Martinez-Lage JF, Sola J, Casas C. Atretic cephalocele: The top of the iceberg. J Neurosurg. 1992:77:230-235.
- Hedlung G. Congenital frontonasal masses: Developmental anatomy, malformations, and MR imaging. Pediatr Radiol. 2006:36(7):647-662.
- Mealey J Jr, Dzenitis AJ, Hockey AA. The prognosis of encephaloceles. J Neurosurg. 1970:32:209-218.
- Volpe JJ. Intracranial hemorrhage: Neural tube formation and prosencephalic development. Neurology of the Newborn. 4th Ed. Philadelphia,PA: WB Saunders; 2001.
Citation
M P, K L, A M, J T, J Q, J N K,. Pediatric Cephaloceles: A Multimodality Review. Appl Radiol. 2020;(5):26-32.
September 1, 2020