Novel Theranostics Approach Cures Colorectal Cancer in Preclinical Study
Images
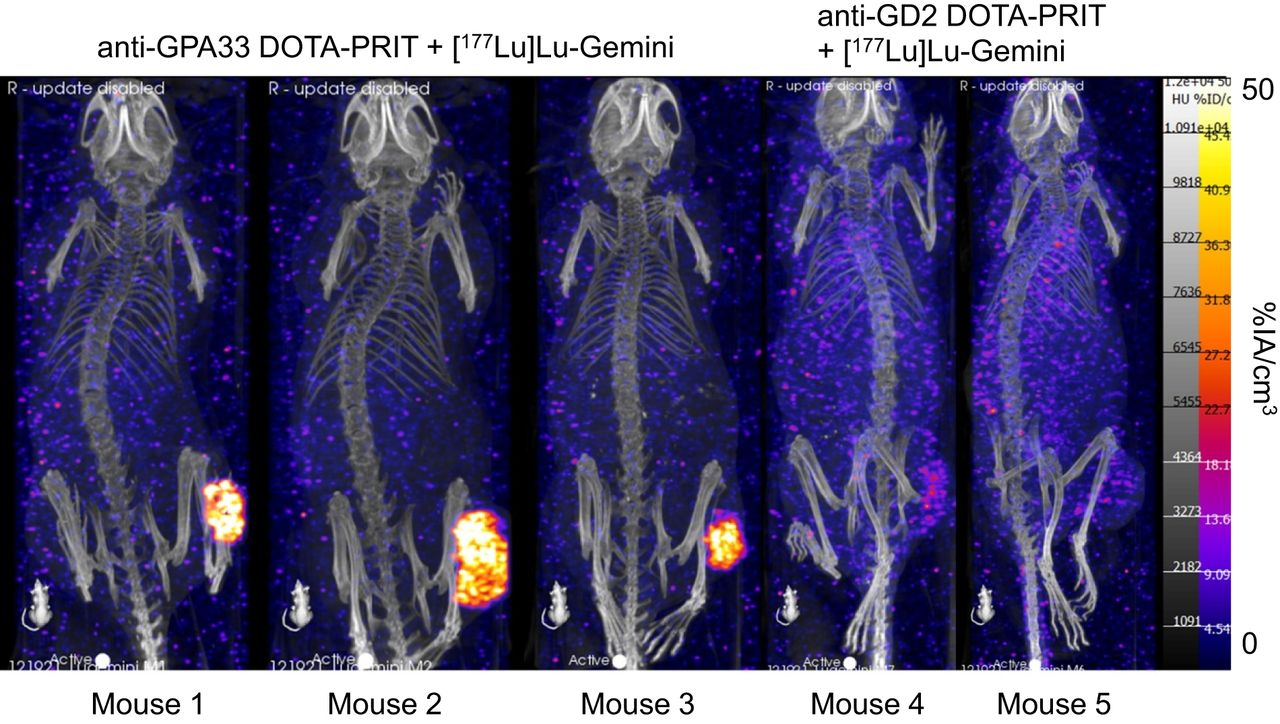
A novel pretargeted radioimmunotherapy (PRIT) approach for colorectal cancer achieved 100% complete response and cured 40% of tumor-bearing mice, according to new research published in The Journal of Nuclear Medicine. By matching the tumor retention time of the targeting radiopharmaceutical with the half-life of the therapeutic radiopharmaceutical, the approach delivered a higher radiation dose while preventing damage to healthy organs and tissues. This study offers significant safety and logistical benefits for both patients and staff.
Effective radiotheranostic treatments face key challenges, such as suboptimal drug delivery and inadequate retention of radionuclides delivered to the target site. A curative dose of the treatment is often unable to be delivered to the target site as too much radiation would reach other healthy tissues. PRIT seeks to overcome these challenges by precisely delivering treatment to the tumor only.
“In our study, we hypothesized that we could improve the efficacy and safety of PRIT by creating a ‘twin’ diagnostic radiopharmaceutical that would match the relatively long physical half-life of 177Lu, a therapeutic radionuclide with a half-life of 6.6 days,” said Sarah M. Cheal, PhD, assistant professor of Biological Chemistry in Radiology at the Molecular Imaging Innovations Institute at Weill Cornell Medical College in New York, New York. “We developed a bivalent radiolabeled molecule called 177Lu-Gemini, also known as ‘the twin’. This molecule enhances the retention of the radiopharmaceutical on a tumor, allowing for a higher dose of treatment to be delivered to the tumor.”
In vitro studies were conducted to determine the specificity of 177Lu-Gemini, and in vivo biodistribution studies in tumor-free mice compared 177Lu-Gemini to the gold standard monovalent 177Lu-DOTA-Bn. A three-step pretargeting approach was then performed with an established DOTA-PRIT regimen in colorectal cancer mouse models.
Biodistribution studies showed that 177Lu-Gemini behaved similarly to 177Lu-DOTA-Bn, with nearly identical blood and whole-body clearance kinetics. Pretargeting 177Lu-Gemini to human colorectal xenografts was also highly effective compared to 177Lu-DOTA-Bn and achieved a 6.5-fold increase in the tumor radiation-absorbed dose. In tumor-bearing mice, a single injection of DOTA-PRIT with 177Lu-Gemini induced complete response in 100% of mice and a histologic cure in 40%. Moreover, a significant increase in survival compared with nontreated controls was also noted, and the maximum tolerated dose not reached.
“The application of 177Lu-Gemini to DOTA-PRIT led to a dramatic reduction in the administered radiation necessary to achieve a histologic cure in our animal study,” noted Steven M. Larson, MD, FACR, FACNM, emeritus professor at Weill Cornell Medical College and emeritus member at Memorial Sloan Kettering Cancer Center, in New York, New York.
“This will potentially translate to cost savings and less radiation hazard to patients and treatment staff.”
He continued, “Due to the modularity of the pretargeted radioimmunotherapy approach, we believe that 177Lu-Gemini can be used to treat many solid tumors, and that the Gemini concept can be adapted for alternative diagnostic and therapeutic radionuclides. This offers a powerful radiotheranostic platform for oncology.”